INTRODUCTION
In this article, we present a single case report involving a lower right molar that includes a clinical and serial radiographic laser-assisted regeneration (LANAP protocol) result over a 13-year time frame.
Key Points Regarding the LANAP Protocol
In the LANAP protocol, no exogenous materials, such as growth factors, bone grafting, and biologics, are used. The true periodontal regeneration achieved in the LANAP protocol is accomplished by using the patient’s own blood proteins that contain stem cells, native growth factors, and blood constituents. The blood is thermally affected in the LANAP protocol in such a manner that the regenerative factors are trapped within a red thrombus that is formed using scientifically determined algorithms of optimal laser operating parameters.
In the LANAP protocol, there is a lack of any wide surgical access outside of the bony housing, a lack of deep dissection into the vestibule or across the palate, and a lack of extensive vertical releasing incisions. The LANAP protocol uses a minimally invasive periodontal flap to enable access under the periosteum and directly to bone for an ostectomy and/or osteotomy. The soft tissues are thereby easily approximated and stabilized without the need to suture with tension to adapt the margins of the flaps together.
Background
Periodontitis is an infectious disease that progressively destroys the alveolar bone, periodontal ligament (PDL), and root cementum that attach the teeth to the bone. Destruction of this attachment apparatus results in the loss of teeth. The ultimate aim of periodontal regeneration techniques is to induce or guide healing to regenerate the morphology back to its original configuration. In order to evaluate a regeneration technique experimentally, a notch is made on the root surface at the bottom of a periodontal pocket to provide a histological landmark for the apical extent of the destruction.1
Periodontal regeneration on a previously diseased tooth root surface is a unique, challenging, and elusive healing event to obtain in humans.FN-1 True periodontal regeneration requires the 3 original components of the periodontal apparatus to arise anew and eventually form into new cementum, a new periodontal ligament, and new alveolar bone.FN-2
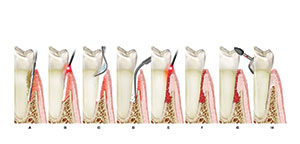 |
| Figure 1. The LANAP protocol: The step-by-step surgical technique is outlined here. (a) Periodontal probing indicates excessive pocket depth. (b) Laser Troughing: Free-runningFN-1 pulsed Nd:YAG laser irradiation is done at short pulse duration or longer, as warranted. Troughing provides visualization of, and access to, the root surface by removing necrotic debris, releasing tension of circumferential periodontal fibers, and controlling hemorrhages. It further defines tissue margins preceding ultrasonic and mechanical instrumentation, preserves the integrity of the mucosa, and aids maintenance of the gingival crest. This technique provides the selective removal of diseased, infected, inflamed, ulcerated pocket epithelium, preserving connective fibrous tissues and rete ridges.21,22 (c) A piezo-electric scaler with specialized tips is used to remove root surface accretions. (d) Bone modification by osteoplasty and/or ostectomy is performed, and angiogenesis is promoted. (e) A second pass with the laser at 150 to 650 µsec pulse duration finishes debriding the pocket; provides hemostasis; and creates a “soft clot,” or red thrombus, resulting in a “closed” biologic thermogenic wound (fibrin clot) from the deep bony defect to the gingival collar. (f) The tissue is adapted against the root surface to create a thin-film clot and stabilize the fibronectin. (g) Occlusal trauma is eliminated with a high-speed handpiece, and mobile teeth are splinted. (h) True periodontal regeneration occurs. |
Kao et al2 is the most recent in a long series of literature and systematic reviews of published methods to achieve periodontal regeneration.3-12 Current approaches include demineralized freeze-dried bone allografts (DFDBAs), guided tissue regeneration (GTR), bone fill with enamel matrix derivatives (EMDs), recombinant human platelet-derived growth factor BB (rhPDGF-BB), and open flap debridement (OFD). Kao et al2 have included a new category of regenerative approaches termed laser-assisted regeneration (LAR), which is identified specifically as the Millennium Dental Technologies LANAP protocol using the PerioLase pulsed Nd:YAG Dental Laser System.FN-3 Based on the review of 2 peer-reviewed studies of human histology following LANAP treatment,13,14 Kao et al2 conclude, “Using the Nd:YAG laser with this [LANAP] procedure, periodontal regeneration is achievable on a previously diseased root surface.”
Data from the first human histology study post-LANAP protocol by Yukna, Carr, and Evans13 in 2007 was submitted to the FDA in 2003. Healing at 3 months clearly showed new attachment and evidence of regeneration. Subsequently, the FDA granted marketing clearance (510(k) No. K030290) on July 26, 2004, for the claim “Laser assisted new attachment procedure (cementum-mediated periodontal ligament new-attachment to the root surface in the absence of long junctional epithelium).” The second, more recent study by Nevins et al14 looked at healing 9 months following LANAP treatment. They concluded, “This report provides evidence that LANAP therapy can induce periodontal regeneration.”14 This data from the human histology study by Nevins et al14 was submitted to the FDA, which granted marketing clearance (510(k) No. K151763) on March 15, 2016, specifically for the PerioLase pulsed Nd:YAG Dental Laser System for the clinical outcome claim:
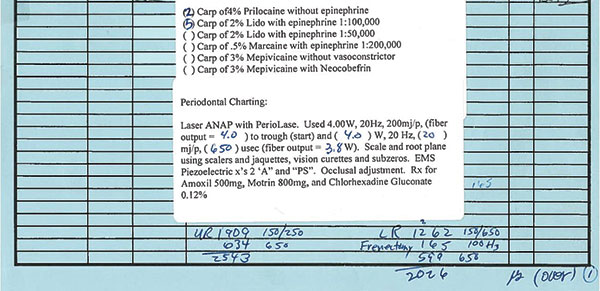 |
| Figure 2. A patient treatment chart shows laser parameters and dosimetries. |
Periodontal regeneration—true regeneration of the attachment apparatus (new cementum, new periodontal ligament, and new alveolar bone) on a previously diseased root surface when used specifically in the LANAP protocol.
In 4 previously published articles in Dentistry Today, the authors presented 10 radiographic case study examples of bone and periodontal ligament (PDL) regeneration around severely compromised, periodontally involved teeth.15-18 It was argued by some skeptics that while those clinical examples are individually impressive, they are isolated and atypical examples of success and not likely to be repeatable. However, in a recent Dentistry Today CE article,19 we reviewed 2 human histology investigations by 2 different groups of investigators (Yukna, Carr, and Evans13 and Nevins et al14). This review combines the histologic findings from those investigations and offers an explanation as to why the case studies had successful outcomes. In this article, we present a single case report to add to the accumulating body of evidence that shows stability over a span of 13 years.
The LANAP protocol is a laser-based periodontal regenerative procedure invented by the lead author and developed specifically for the treatment of moderate-to-advanced periodontitis. It was patterned, conceptually, after the excisional new attachment procedure (ENAP)20 to separate the epithelium from the underlying connective tissue dermis and to selectively vaporize and disrupt diseased, infected, inflamed, and necrotic tissue from the connective tissue.21,22 Lasers are not used as replacements for the scalpel. Scalpels cannot approach the kind of differential selectivity needed to separate thin, discrete tissue types.
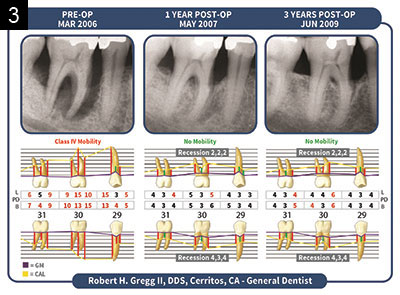 |
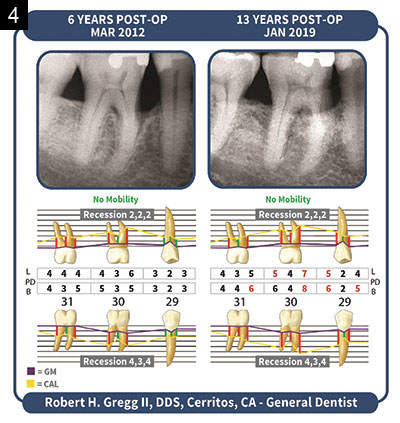 |
| Figures 3 and 4. Radiographs over a period of 13 years display where we have conducted a preliminary radiographic density analysis. There is clear evidence for an increase in the density of interproximal alveolar bone following the LANAP Protocol. Formation of new compact bone appears adjacent to the root surfaces (cribiform plate). This, along with human histology, is evidence for bone regeneration of the alveolar crest. |
The LANAP protocol, which retains the original 26 sequential steps, was initially referred to as Laser-ENAP,15,16 then laser periodontal therapy (LPT) due to FDA insistence over possible confusion with scalpel ENAP. The human histology study performed by Yukna, Evans, et al23 in 2003 was the basis for the novel FDA 510(k) clearance for the laser-assisted new attachment procedure in 2004.
The LANAP protocol is, by definition, a one-time full-mouth treatment protocol as a complete replacement alternative to osseous resective periodontal surgery and/or scaling and root planing. The LANAP protocol is completed in a one-half mouth treatment of 2 quadrants, followed up with a second one-half mouth treatment of the other 2 quadrants after a few days, but no more than one week apart. The patient has antibiotic coverage between the separate treatment appointments. The patient can also have his or her full mouth treated (4 quadrants) in one visit. There are no subsequent treatment visits after the 4 quadrants are initially treated. Recurring periodontitis, if any, may be retreated with the full-mouth LANAP protocol years later if the disease complex returns.
Numerous other advantages of the LANAP protocol include improved patient treatment acceptance; significantly reduced patient appointments; reduced patient postoperative morbidity; tissue-height preservation; intraoperative hemostasis; and reduced post-op pain, bleeding, swelling, and infections. The procedure combines the best aspects of laser soft-tissue surgery with well-established principles of periodontal disease reversal.
Materials and Methods
A free-running (FR) pulsed Nd:YAG laser (PerioLase MVP-7 [Millennium Dental Technologies]) was used for these patients.FN-4 This device provided an improvement over all existing FR pulsed Nd:YAG laser systems by having the availability of 7 multi-variable, operator-controlled pulse durations.FN-5 Troughing around the tooth was typically done with a “short pulse,” having a duration of 100 to 150 µsec, although intraoperative clinical determinants allow for longer pulse durations to be used as necessary. The pulse energy was set to 160 to 200 mJ, and the repetition rate was 20 Hz, giving an average power of 3.2 to 4.0 W. Again, intraoperative clinical determinants and fiber diameters necessitate the need to adjust the average wattage up or down as necessary and as indicated. The parameters for the hemostasis, or “long pulse,” used to finish the procedure range from a duration of 150 to 650 µsec. Average powers displayed on the console are confirmed using a built-in power meter.FN-6 (Please note: Exceeding an average power of 4.0 W is not recommended for anyone except the most experienced or expertly trained laser user.FN-7)
 |
 |
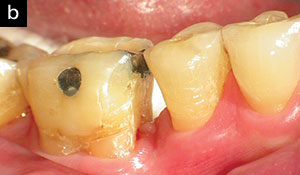 |
| Figure 5. Lower right molar No. 31, 6-year clinical postoperative image. Note the intentional and complete removal of the distal amalgam and the majority of the central occlusal amalgam as part of the LANAP protocol in 2006. Proximal teeth “guard” the molar from occlusal trauma. Bleeding on probing was noted on the mesial, and inflammation was treated with scaling and root planing and laser pocket disinfection. |
Another advantage of this laser system is the display of total energy delivered to the oral tissues during the procedure. This value is essential in determining the light dose (Joules per millimeter pocket depth [J/mm PD]).FN-8 The light dose is calculated by dividing the total energy delivered by the sum of the depths of all pockets to safely dose the tissue with the appropriate range of light energy, which will safely range between 12 to 17 J/mm PD (Figure 1).
The bactericidal effects of the FR pulsed Nd:YAG laser,24-27 plus the intraoperative use of topical antibiotics, are designed for the reduction of microbiotic pathogens (antisepsis) within the periodontal pockets and bony defects and the surrounding tissues. The red thrombus is stabilized, and occlusal trauma is eliminated to promote true periodontal regeneration. Oral hygiene is stressed, and continued periodontal maintenance is scheduled.
The desired result is to achieve true periodontal regeneration (ie, new bone, PDL, and, cementum) to the root surface, thereby decreasing pocket depth by 50% in more than 90% of patient pockets through regeneration, not amputation.
CASE REPORT
Patient: A 59-year-old male (Figures 2 to 5).
February 21, 2006: Consult.
Chief Concern: To avoid conventional surgery for the treatment of his bleeding gums.
Medical: The patient reported no health conditions or medications. He developed brain cancer in 2009 and Parkinson’s disease in 2012.
Consultation and Exam: The patient had heard of LANAP treatment from a television report. A periodontal risk assessment and prognosis form were completed with the patient. Informed consent was given after reviewing the alternatives, risks, and benefits with the patient, including no treatment. He declined alternative treatment options.
The LANAP protocol was reviewed with the patient, as well as the full-mouth treatment sequence of 2 visits, each one treating one-half of the mouth, plus a post-op assessment. The patient was informed that the LANAP protocol requires “spot grinding” of the teeth and any crowns and, separately, that laser treatment is not a “magic wand.” Laser safety was reviewed with the patient.
Patient risk assessment/patient report:
- Hygiene appointment 3 years prior
- No history of periodontal surgery
- Diagnosis of periodontal disease
- Recommendation for conventional scalpel and suture surgery
- Gingival tissues bleed “badly”
Clinical findings:
- Soft tissues were red and inflamed
- A presence of generalized calculus
- Gingival recession and mucogingival defects
- Light probing revealed generalized 6-mm-plus probe depths and bleeding on probing
- A Class IV vertically compressible mobility of tooth No. 30
Diagnosis: Generalized, severe, chronic, adult periodontitis; Case Type IV.
Prognosis: Guarded.
March 13, 2006: The right upper/lower quadrants were treated.
March 20, 2006: The left upper/lower quadrants were treated.
Bone Density
Clinical comparison of the LANAP Protocol pretreatment with post-radiographs indicates the formation of new bone. We have not completed the analysis that will indicate the frequency of this occurrence, but we can suggest that it is frequent among LANAP protocol patients. In a previous study, we presented 40 patient outcomes where sampled sites indicate that 100% of the cases demonstrated increased bone density by an average of 38%.28 Crestal/horizontal height had clearly increased, but the quality and density of the bone was especially noteworthy. Also evident was new cortical crestal bone and lamina dura and a defined PDL space.
Light Dosimetry
Critical to patient safety and treatment efficacy is the ability to record and document the Joules per millimeter pocket depth (J/mm PD) of the quadrants of teeth and/or of a specific tooth. This allows the clinician to maintain the proper exposure of infrared thermal laser energy to oral tissues and eliminate adverse treatment outcomes of teeth, soft tissue, and bone.
This patient received only one full-mouth treatment of the LANAP protocol in 2006 and received no repeat treatments with the LANAP protocol.
As discussed previously in the Materials and Methods section, light dose (Joules per millimeter pocket depth) was calculated for this patient. In the 2 quadrants reported in this article, there were 6 teeth per quadrant. Their values are shown in Table 1.
CLOSING COMMENTS
The results reported here in no way suggest that other non-laser methodologies for treating periodontal bony defects (eg, guided bone regeneration) do not also lead to periodontal regeneration (eg, Straumann Emdogain).However, the LANAP protocol is a full-mouth protocol vs a site-specific regenerative treatment.
The results of real-world case results, confirmed by the research gold standard of human histology, confirm that using an FR pulsed Nd:YAG laser with optimized operating parameters, together with appropriately applied treatment algorithms, provides an additional benefit of true periodontal regeneration and reduced pocket depths over what conventional scaling and root planing, or osseous surgery alone, can achieve.FN-9 Furthermore, the LANAP protocol is another approach to minimally invasive surgical therapies, a minimally invasive surgical approach that may offer advantages in the regeneration of defects, and may be appropriate for multiple defects as a first line of management.2
Acknowledgments:
We thank John Sulewski for manuscript review, editing, and citations. We also thank Rachel Moody; Jennifer Iglesias; Veronica Santa; Vanessa Grimes; and Marika Lockhart, RDH, for radiograph and periodontal-charting graphic presentations.
Footnotes
- “Currently, osseous grafting and guided tissue regeneration (GTR) are the 2 techniques with the most histologic documentation of periodontal regeneration. Other regenerative therapies have also provided a promising potential for significantly improving clinical parameters and demonstrating substantial “fill” of treated defects. However, only limited histologic evidence of true regeneration has been demonstrated with the majority of these therapies.”1
- “Regeneration refers to the reproduction or reconstitution of a lost or injured part, in contrast to repair, which describes healing of a wound by tissue that does not fully restore the architecture or the function of the original part. Periodontal regeneration is defined histologically as regeneration of the tooth’s supporting tissues, including alveolar bone, periodontal ligament, and cementum over a previously diseased root surface. New attachment is defined as the union of connective tissue or epithelium with a root surface that has been deprived of its original attachment apparatus. This new attachment may be epithelial adhesion and/or connective tissue adaptation or attachment and may include new cementum. It is to be distinguished from reattachment, which describes the reunion of epithelial and connective tissue with a root surface. Bone fill is defined as the clinical restoration of bone tissue in a treated periodontal defect. Bone fill does not address the presence or absence of histologic evidence of new connective tissue attachment or the formation of new periodontal ligament.”1
- “…this technique is intriguing in that it is another approach to minimally invasive surgical therapies as reviewed by Cortellini.29 A minimally invasive surgical approach may offer advantages in regeneration of defects in the aesthetic zone in which minimal soft tissue change is required. Additionally, because of the minimally invasive nature and expendable surgical materials required, this approach may be appropriate for multiple defects as a first line of management.”2
- Free Running (FR) is the measure of the time duration of a single pulse in 10-6 seconds, or millionths of a second or microseconds (µsec). This allows for high peak powers in the order of 1,000 to 3,000 W per pulse and pulse intervals that are 500 or more times longer than the pulse “on” time.
- Pulse Duration can be measured several ways, depending on whether the pulse is digital or analog. Digital pulse durations are qualitatively and quantitatively different from analog pulse durations. An analog pulse has a Gaussian profile (ie, a sine wave), whereas the digital pulse is square. Digital pulse durations are more accurately measured than analog ones since the shape of the area measured is a discrete area vs an alternating wave front. The convention used here is known as full width/half max. That is the pulse time (duration) in microseconds measured the full width on the x-axis (width) of an oscilloscope at one-half the maximum of the y-axis.
- Power (Watts): The rate of doing work. It is critical to accurate communications of dosimetry that therapeutic power delivered to tissue be confirmed through measurement at the fiber tip with a calibrated power meter, as the power can vary as much as 30% or more from the power settings displayed on the console of any laser device. A power meter (PM10-19AW [Coherent, Inc]) was used in the case study presented.
- Caution: Laser dosimetry described in this paper is not recommended unless the practitioner is well-trained and experienced. Exceeding the laser parameters or overtreating the large defects described for these cases may lead to prolonged healing, tissue and tooth loss, and other complications.
- Light dose (Joules per millimeter pocket depth) is similar to drug dose (milligrams per kilogram of body weight) in that light dose defines the concentration of laser energy at the treatment site in a similar manner as drug dose defines the concentration of a drug in the tissues. Light dose is a very useful parameter inasmuch as certain clinical outcomes of laser surgery (eg, adverse effects) are dose-dependent.
- Other Nd:YAG laser devices: One cannot extrapolate to other laser devices or other treatment protocols that have not defined their protocols, operating parameters, treatment algorithms, or healing events absent human histology.
References
- Wang HL, Greenwell H, Fiorellini J, et al; Research, Science and Therapy Committee. Periodontal regeneration. J Periodontol. 2005;76:1601-1622.
- Kao RT, Nares S, Reynolds MA. Periodontal regeneration—intrabony defects: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86(suppl 2):S77-S104.
- Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, et al. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8:227-265.
- Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003;8:266-302.
- Needleman I, Tucker R, Giedrys-Leeper E, et al. Guided tissue regeneration for periodontal intrabony defects—a Cochrane systematic review. Periodontol 2000. 2005;37:106-123.
- Aichelmann-Reidy ME, Reynolds MA. Predictability of clinical outcomes following regenerative therapy in intrabony defects. J Periodontol. 2008;79:387-393.
- Koop R, Merheb J, Quirynen M. Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol. 2012;83:707-720.
- Esposito M, Grusovin MG, Papanikolaou N, et al. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur J Oral Implantol. 2009;2:247-266.
- Sculean A, Nikolidakis D, Schwarz F. Regeneration of periodontal tissues: combinations of barrier membranes and grafting materials—biological foundation and preclinical evidence: a systematic review. J Clin Periodontol. 2008;35(8 suppl):106-116.
- Esposito M, Coulthard P, Thomsen P, et al. Enamel matrix derivative for periodontal tissue regeneration in treatment of intrabony defects: a Cochrane systematic review. J Dent Educ. 2004;68:834-844.
- Giannobile WV, Somerman MJ. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol. 2003;8:193-204.
- Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL. Regeneration of periodontal tissue: bone replacement grafts. Dent Clin North Am. 2010;54:55-71.
- Yukna RA, Carr RL, Evans GH. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent. 2007;27:577-587.
- Nevins ML, Camelo M, Schupbach P, et al. Human clinical and histologic evaluation of laser-assisted new attachment procedure. Int J Periodontics Restorative Dent. 2012;32:497-507.
- Gregg RH, McCarthy DK. Laser ENAP for periodontal bone regeneration. Dent Today. 1998;17:88-91.
- Gregg RH, McCarthy DK. Laser ENAP for periodontal ligament regeneration. Dent Today. 1998;17:86-89.
- Gregg RH II, McCarthy D. Laser periodontal therapy: case reports. Dent Today. 2001;20:74-81.
- Gregg RH II, McCarthy D. Laser periodontal therapy for bone regeneration. Dent Today. 2002;21:54-59.
- Gregg RH II, Gregg DM. Laser-assisted periodontal regeneration and human histology. Dent Today. 2019;38:70-74.
- Yukna RA, Bowers GM, Lawrence JJ, et al. A clinical study of healing in humans following the excisional new attachment procedure. J Periodontol. 1976;47:696-700.
- Gold SI, Vilardi MA. Pulsed laser beam effects on gingiva. J Clin Periodontol. 1994;21:391-396.
- Ting CC, Fukuda M, Watanabe T, et al. Morphological alterations of periodontal pocket epithelium following Nd:YAG laser irradiation. Photomed Laser Surg. 2014;32:649-657.
- Yukna RA, Evans GH, Vastardis S, et al. Laser-assisted periodontal regeneration in humans. Poster presented at: 81st General Session of the International Association for Dental Research; June 27, 2003; Goteborg, Sweden. Abstract 1735.
- Moritz A, Schoop U, Goharkhay K, et al. The bactericidal effect of Nd:YAG, Ho:YAG, and Er:YAG laser irradiation in the root canal: an in vitro comparison. J Clin Laser Med Surg. 1999;17:161-164.
- Whitters CJ, MacFarlane TW, MacKenzie D, et al. The bactericidal activity of pulsed Nd-YAG laser radiation in vitro. Lasers Med Sci. 1994;9:297-303.
- Cobb CM, McCawley TK, Killoy WJ. A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. J Periodontol. 1992;63:701-707.
- Neill ME, Mellonig JT. Clinical efficacy of the Nd:YAG laser for combination periodontitis therapy. Pract Periodontics Aesthet Dent. 1997;9(6 suppl):1-5.
- Gregg RH II, McCarthy DK. Eight-year retrospective review of laser periodontal therapy in private practice. Dent Today. 2003;22:74-79.
- Cortellini P. Minimally invasive surgical techniques in periodontal regeneration. J Evid Based Dent Pract. 2012;12(3 suppl):89-100.
Dr. Robert H. Gregg II graduated from Georgetown University Dental School in 1984. He maintains a private group practice in Cerritos, Calif, that has been in the same location for 33 years. Dr. Gregg began using lasers in clinical practice in 1990 and remains at the forefront of dental laser technology, research, innovation, and clinical adoption. He invented the LANAP and LAPIP protocols while founding Millennium Dental Technologies, Inc, to provide patients with minimally invasive, regenerative treatment options and hope for hopeless teeth. He also serves as the president of the Institute for Advanced Laser Dentistry (IALD) and is an adjunct associate professor at the Rutgers School of Dental Medicine (RSDM). He can be reached via email at rgregg@lanap.com.
Disclosure: Dr. Robert Gregg II is president of Millennium Dental Technologies, Inc.
Dr. Dawn M. Gregg is a fourth-generation dentist, a Member of the Omicron Kappa Upsilon Dental Honor Society, and a graduate of the University of California, Los Angeles School of Dentistry. Dr. Gregg serves as CEO and training director for the IALD and is an adjunct associate professor at RSDM. She is responsible for ongoing initiatives with dental curricula where LANAP and LAPIP protocols are being taught, and she orchestrated a multi-center clinical study in the role of LANAP clinical study monitor. She can be reached via email at dgregg@lanap.com.
Disclosure: Dr. Dawn Gregg is vice president of operations of Millennium Dental Technologies, Inc.
Related Articles
Importance of the Quality of Tissue Supporting Dental Implants
LANAP Protocol Gains US Patent
University of Kentucky Adopts LANAP and LAPIP Protocols











