INTRODUCTION
Bone preservation has become of paramount importance when tooth extractions are performed and full-arch dentures are indicated. Recently, research suggests that the physical and chemical composition of tooth-derived bone grafting materials makes them efficacious as an alternative bone grafting material in extraction sockets. This article discusses the characteristics of tooth-derived bone grafting materials, as well as the benefits of the chairside Smart Dentin Grinder device (KometaBio). A case presentation demonstrates the use of the device in the treatment of a patient who required extraction of her remaining maxillary dentition, immediate bone grafting, and delivery of an interim healing full-arch removable denture.
Numerous advancements have been made to enhance our patients’ oral health, including the prevention and treatment of periodontal disease as well as risk assessment and the management of caries disease. Unfortunately, many individuals still remain for whom the extraction of all teeth in one or both arches will be necessary and the replacement of lost teeth with an immediate full-arch removable prosthesis will be the most affordable and prudent treatment.1 However, even when undertaken in the most atraumatic manner possible, tooth extraction results in alterations to the hard and soft tissues that can compromise the fit and function of the prosthesis and contribute to aesthetic deficits and further bone resorption and soft-tissue collapse.2 For this reason, bone preservation has become of paramount importance when tooth extractions are performed and, in particular, when immediate full-arch dentures are indicated.3
Over the years, a variety of bone grafting materials has been utilized in combination with different ridge preservation and augmentation techniques. These have included allografts (ie, derived from human cadaver bone), xenografts (ie, derived from different species, such as bovine, porcine, equine, etc), autologous material (ie, derived from an individual’s own bone), and autogenous dentin (ie, derived from a patient’s extracted teeth).
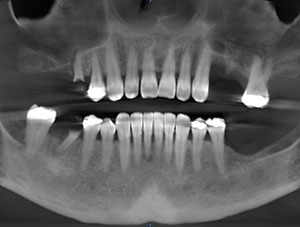 |
| Figure 1. Preoperative radiograph of a woman who presented with severely mobile maxillary dentition that was poorly supported by deficient alveolar bone. The extraction of these teeth and their replacement with a full-arch removable prosthesis was planned. |
Most recently in the literature, the physical and chemical composition of tooth-derived bone grafting materials has been studied. Reports indicate that the osteoconductive properties of these materials make them efficacious as an alternative bone grafting material.4,5 Additionally, because the materials can be produced from freshly extracted teeth and processed chairside using a dentin grinder (eg, the Smart Dentin Grinder), researchers have also cited this alternative’s availability and low cost as compared to other commercially available bone grafting materials.5
Contributing to dentin’s favorable use as a bone grafting material is its natural, bioactive, and autologous structure, which is virtually identical in composition to bone. When placed into an extraction socket, the tooth-derived dentin bone graft material does not resorb quickly but instead attracts osteoprogenitors from the site, essentially undergoing ankylosis (ie, fusion) with the bone.5 The fused bone/dentin matrix then remodels slowly, which in turn helps to maintain aesthetics. Still, more importantly, the matrix promotes new bone formation at the site due to the autologous nature of the graft.6,7 In fact, after 15 to 18 months of remodeling, the dentin particulate is usually no longer visible in the bone, and healing occurs without signs of inflammatory response.5-7
A Chairside Approach
The Smart Dentin Grinder is a chairside device that converts a patient’s own tooth or teeth into autologous grafting material for placement back into the same patient. The device features a single-use grinding chamber that pulverizes the tooth into granules, then subsequently sorts them into 2 size ranges (eg, between 300 and 1,200 µm and smaller than 300 µm). Once the grinding procedure is finished, the granules are sterilized using chemical sterilization, although autoclave sterilization is also possible.
According to the manufacturer, testing has demonstrated that more than 98% of an original tooth processed by the Smart Dentin Grinder is ground, sorted, and recovered in the unit’s compartments. The dentist can use all granules that are produced or choose one size range if preferred. Additionally, the device has demonstrated effectiveness at grinding teeth consistently, regardless of variations in tooth morphology, as well as achieving consistent particulate sizing based on the sieves that sort the graft material.
The Smart Dentin Grinder offers 2 protocols: one for mineralized dentin grafts and a separate protocol for demineralized dentin grafts. The demineralized dentin graft protocol produces a combination of mineralized and demineralized particulate, which helps to expose the organic portion earlier while still maintaining an excellent, slow-resorbing mineral scaffold.
Interestingly, the Smart Dentin Grinder does not produce heat during the standard 3-second protocol (ie, the pulverization of the tooth structure, as opposed to grinding, cutting, or drilling). Therefore, there is no heat impact on the organic portion of the material, which is protected by the tooth’s mineralization.
The following case demonstrates the chairside use of the Smart Dentin Grinder when treating a patient who required extraction of her remaining maxillary dentition, im-mediate bone grafting, and delivery of an interim healing full-arch removable denture.
CASE REPORT
Diagnosis and Treatment Planning
A 57-year-old woman presented with severely mobile maxillary dentition (ie, teeth Nos. 3 to 9 and 11 to 13) that were poorly supported by deficient alveolar bone (Figure 1). Her other maxillary dentition was already missing, and a thorough intraoral and radiographic examination revealed significant periodontal disease.
Following a comprehensive discussion with the patient about her dentition’s hopelessness, it was determined that the extraction of all remaining maxillary teeth was prudent, and her teeth would be replaced with a full-arch, removable prosthesis. Although an implant-retained/supported overdenture was discussed with the patient as a more stable prosthetic solution, the patient declined this option due to health concerns (ie, she recently received a cancer diagnosis).
 |
 |
| Figure 2. The extracted teeth were prepared for grinding by using a high-speed handpiece to remove any cavities or foreign material, ensuring that only pristine tooth structure remained. | Figure 3. Four of the prepared teeth were placed inside the Smart Dentin Grinder (KometaBio) chamber and adjacent to the blades. |
 |
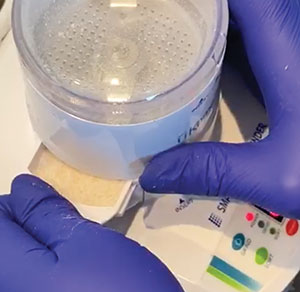 |
| Figure 4. After closing the chamber cap and twisting and clicking it into place, the grinding time was set to the recommended 3 seconds. | Figure 5. Once grinding was finished, the particles were sorted for 10 seconds, and no large particles were left in the grinding chamber. |
 |
 |
| Figure 6. The ground tooth particles were sorted into 2 different chambers. Here, the top-drawer compartment shows particles of between 300 to 1,200 µm. | Figure 7. The remaining extracted teeth were then also ground according to the previously described protocol. |
 |
 |
| Figure 8. View of the particles generated by grinding the remaining extracted teeth. | Figure 9. The sterile container was filled with a dentin cleanser, which was poured over and completely covered the dentin particles. |
 |
 |
| Figure 10. A sterile gauze was used to dehydrate the dentin cleanser solution. | Figure 11. The EDTA and dentin particles were dehydrated using sterile gauze. |
Additionally, due to the extent of bone loss and lack of periodontal support, the patient was advised that bone grafting of the extraction sockets would be necessary to ensure the comfortable fit and proper function of her prosthesis. In fact, the literature cites numerous physical effects of tooth loss that ultimately contribute to the instability of removable denture prostheses, including the loss of hard and soft tissue due to bone resorption. This can lead patients to experience difficulty when speaking and eating, as well as force them to use their cheek muscles, tongues, and lips to keep their dentures in place.8-10 However, the patient was advised that autogenous bone grafting material produced by grinding her extracted teeth (eg, with the Smart Dentin Grinder) would be placed immediately into the extraction sockets to preserve aesthetics, prevent bone resorption, and avoid the need for future grafting procedures that could be more complicated and costly.
Extraction
Local anesthesia was administered to infiltrate both the buccal and palatal areas. Care was taken to atraumatically extract teeth Nos. 3 to 9 and 11 to 13 with forceps. A simple extraction technique was performed with care taken to ensure that 3 or more bony walls (ie, lingual, mesial, distal, apical) remained intact. This would facilitate augmentation and preservation of the edentulous sites and the bony ridge overall.
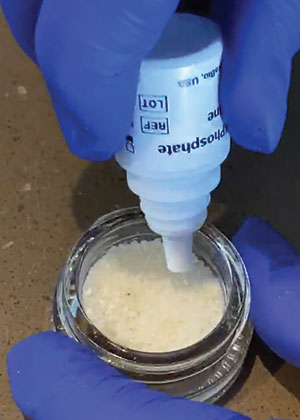 |
| Figure 12. A phosphate-buffered saline wash was then placed into the sterile mixing container to completely cover the particulate. |
Dentin Autologous Bone Graft Material
Following the extraction of teeth Nos. 3 to 9 and 11 to 13, the teeth were prepared for grinding. Using a high-speed handpiece, any cavities or foreign material was removed, ensuring that only pristine tooth structure remained (Figure 2); it was not necessary to decoronate or remove the enamel. The teeth were then thoroughly dried using an air syringe.
Four of the prepared teeth were then placed inside the grinding chamber of a chairside dentin grinder (eg, Smart Dentin Grinder) and adjacent to the blades (Figure 3), as only 4 teeth can be ground at a time. The chamber cap was then closed, twisted, and clicked into place, and the grinding time was set to the recommended 3 seconds (Figure 4). Once grinding was done, the particles were sorted for 10 seconds, with no large particles left in the grinding chamber (Figure 5). Note that particles were sorted into 2 different chambers: a top-drawer compartment contained particles of between 300 and 1,200 µm (Figure 6); a bottom-drawer compartment contained particles smaller than 300 µm.
The ground tooth particles were transferred to a sterile container/mixing dish for cleaning and preparation prior to placement in the extraction sockets. The remaining extracted teeth were then also ground according to the previously described protocol (Figures 7 and 8), and the particles were added to the sterile container/mixing dish. When combined, the grinding of all the extracted teeth produced a significant volume of tooth particle material for use in bone grafting the extraction sockets. In fact, depending upon the tooth, the Smart Dentin Grinder typically generates between 2.5 and 3 times the volume of a tooth, which equates to between 0.8 cc and 3 cc of dentin graft.
To clean the dentin graft particles, the sterile container was filled with a dentin cleanser, which was poured over the dentin particles to completely cover them (Figure 9). After resting for 5 minutes at room temperature, a sterile gauze was used to dehydrate the dentin cleanser solution (Figure 10).
Next, EDTA was added to the dentin graft particles, allowed to sit for 2 minutes, and then dehydrated using a sterile gauze (Figure 11). To complete the preparation process, a phosphate-buffered saline wash (Figure 12) was then placed into the sterile mixing container to completely cover the particulate, a sterile instrument was used to mix the material, and a new sterile gauze was used for dehydrating the graft material.
Bone Graft Placement/Healing
The dentin-derived bone graft material was immediately placed into the extraction sockets using hand instrumentation, and the material was stabilized and secured with a resorbable collagen membrane (Salvin Dental). Primary closure was achieved using gut sutures. A full interim healing denture was then placed.
CONCLUSION
Autologous-sourced bone graft materials, such as the tooth-derived material available using the Smart Dentin Grinder, help promote non-disruptive healing when tooth extractions and immediate bone grafting are required. In addition to being one of the few graft types that induces bone regeneration, dentin bone graft material contributes to a durable scaffold that resorbs slowly, so woven bone material can sufficiently mature.11 For this case, it was a cost-effective and conservative means to provide the bone grafting treatment that the patient required in the most biocompatible manner possible.
References
- Yeung C, Leung KCM, Yu OY, et al. Prosthodontic rehabilitation and follow-up using maxillary complete conventional immediate denture. Clin Cosmet Investig Dent. 2020;12:437-445. doi:10.2147/CCIDE.S271304
- Caplanis N, Lozada JL, Kan JY. Extraction defect assessment, classification, and management. J Calif Dent Assoc. 2005;33(11):853–63. https://pubmed.ncbi.nlm.nih.gov/16463907/.
- Rignon-Bret C, Hadida A, Aidan A, et al. Efficacy of bone substitute material in preserving volume when placing a maxillary immediate complete denture: study protocol for the PANORAMIX randomized controlled trial. Trials. 2016;17(1):255. doi:10.1186/s13063-016-1380-7
- Khanijou M, Zhang R, Boonsiriseth K, et al. Physicochemical and osteogenic properties of chairside processed tooth derived bone substitute and bone graft materials. Dent Mater J. 2021;40(1):173-183. doi: 10.4012/dmj.2019-341
- Mazor Z, Horowitz RA, Prasad H, et al. Healing dynamics following alveolar ridge preservation with autologous tooth structure. Int J Perio Restor Dent. 2019;39(5):697-702. doi:10.11607/prd.4138
- Fernandez de Grado G, Keller L, Idoux-Gillet Y, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng. 2018;9:2041731418776819. doi:10.1177/2041731418776819
- Andrade C, Camino J, Nally M, et al. Combining autologous particulate dentin, L-PRF, and fibrinogen to create a matrix for predictable ridge preservation: A pilot clinical study. Clin Oral Investig. 2020;24(3):1151–60. doi:10.1007/s00784-019-02922-z
- Vogel RC. Implant overdentures: a new standard of care for edentulous patients current concepts and techniques. Compend Contin Educ Dent. 2008;29(5):270-6; quiz 277-8. PMID: 18795644. https://pubmed.ncbi.nlm.nih.gov/18795644/.
- Henry K. Q&A on the future of implants. Dental Equipment and Materials. September/October 2006.
- Rossein KD. Alternative treatment plans: implant supported mandibular dentures. Inside Dentistry. 2006;2:42-43. https://www.aegisdentalnetwork.com/id/2006/08/clinical-treatment-options-alternative-treatment-plans-implant-supported-mandibular-dentures.
- Sánchez-Labrador L, Martín-Ares M, Ortega-Aranegui R, et al. Autogenous dentin graft in bone defects after lower third molar extraction: a split-mouth clinical trial. Materials. 2020;13(14):3090. doi:10.3390/ma13143090
Dr. Simos received his DDS degree from Loyola University in Chicago and maintains a private practice, Allstar Smiles, in Bolingbrook and Ottawa, Ill. He is the founder and president of the Allstar Smiles Learning Center, teaches postgraduate courses in comprehensive restorative dentistry, and is a recognized leader in cosmetic and restorative dentistry. In addition, he lectures throughout the country and is an internationally published author on the use of today’s innovative techniques and materials in dentistry. He can be reached at sam.s@allstarsmiles.com.
Disclosure: Dr. Simos reports no disclosures.
Related Articles
Electrical Bone Mill Unit Regenerates Bone in 8 Minutes
Three Impression Material Classifications: A Comparison
Don’t Undervalue the Overdenture! A Secure and Affordable Option











