Tissue engineering is defined as any attempt to facilitate regeneration of tissues in the body by combining appropriate biologic mediators and matrices.1 Many procedures performed by periodontists are included under this definition. Soft-tissue grafting procedures, periodontal regenerative procedures, and surgical procedures with the administration of recombinant growth factors or amelogenin-like factors fall within the definition of tissue engineering.2 The surgical wound contains a scaffold of cells and signaling molecules.3 In the appropriate environment with an adequate blood supply and control of both bacterial contamination and other environmental factors, regeneration of the periodontium can be achieved (Figure 1).4 By understanding the biologic principles of wound healing and regenerative medicine, clinicians can optimize the results of these procedures.
Advances in regenerative techniques are revolutionizing periodontal therapy. Periodontics once relied on resective techniques to establish periodontal health. These techniques often result in aesthetic deformities, with long clinical crowns, receded gingival tissues, and increased risk for root sensitivity and root caries. Periodontics now utilizes regenerative techniques to offer surgical approaches that actually conserve tissue contour and aesthetics. The combination of newer procedures and biologically active materials to stimulate the regenerative response is changing the approach and patient outcomes of periodontal treatment.
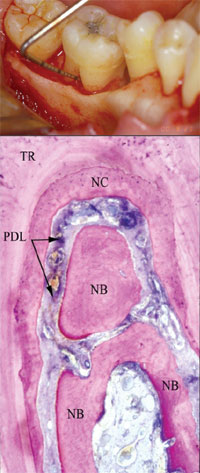 |
| Figure 1. This grade II furcation defect was successfully treated with the growth factor rhPDGF-BB delivered in an allogeneic matrix, as evidenced by the histologic results. Note new bone (NB), new cementum (NC), and periodontal ligament (PDL). (Image reprinted with permission of the Journal of Periodontology.) |
One must combine the latest technologies with surgical advances to improve patient care. The introduction of the connective tissue graft 20 years ago allowed periodontists to provide patients with excellent functional and aesthetic results.5 It has been documented in the literature that this is a highly predictable procedure and is the standard of care for root coverage therapy.6 Even with advanced root exposure, current advances like the implementation of techniques such as modified double pedicle flap design and amelogenin-like factors can significantly enhance grafting procedures.7,8 The goal of these advances is to provide root coverage that will be stable over time and will meet the aesthetic goal of not being easily identified as having been surgically treated.
Currently, in clinical practice a high percentage of oral plastic surgical procedures can benefit from the use of amelogenin-like factors or growth factors to enhance therapeutic results. These include periodontal regeneration procedures, root coverage with connective tissue grafting, socket preservation, and implant site development with ridge grafting or sinus grafting. These procedures can either apply new therapeutic biomaterials as a novel therapy or utilize growth factors as an adjunctive agent to enhance a current technique. The following cases demonstrate the utilization of regenerative therapies with tissue engineering.
CASE 1
This patient presented with a maxillary right canine with advanced gingival recession that was treated with a free gingival graft approximately 15 years ago (Figure 2a). The graft was successful in preventing further gingival recession but did not meet the patientÃs aesthetic needs. She had a high smile and was concerned with the visible recession presenting on both maxillary canines.
The use of a connective tissue graft combined with a modified double pedicle technique, enamel matrix derivative (EMD [Straumann]), and a coronally advanced flap offered a predictable treatment option.
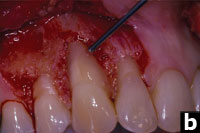
The site was treated by surgically accessing the dehisced root surface (Figure 2b). The root surface was aggressively root planed with hand instruments, and any previously placed composite restoration was removed. The root surface was œconditioned” with tetracycline prior to and a topical, neutral root surface conditioner (PrefGel [Straumann]) after root planing. A connective tissue graft was harvested from the maxillary right palate. Primary closure of the donor site was attained. EMD was applied to the cleaned and dried root surface, to the recipient surgical site, and also to the palatal wound.
 |
 |
|
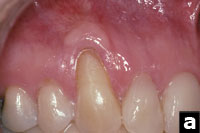
Figures 2a and 2b. A high smile line and clefting of the gingiva present an aesthetic challenge. The osseous defect is exposed. |
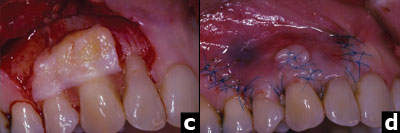 |
| Figures 2c and 2d. The root surface is treated with tetracycline paste, EMD, and a connective tissue graft. The cleft of receded gingiva is repaired with 7-0 polypropylene sutures. |
 |
|
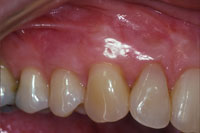
Figure 2e. At 1-year postoperatively there is near total root coverage and a very good aesthetic result. |
The connective tissue graft was sutured at the level of the CEJ using sling sutures (6-0 chromic gut) with the knots tied on the palate to keep them away from the graft. The repaired buccal flap was then coronally advanced to achieve primary closure over the graft, and sutured tension-free with sling sutures and interrupted sutures at the vertical incisions (Figures 2c and 2d).
Six months postoperatively a gingivoplasty was performed to smooth the area of the old free gingival graft. Although the high smile created a high aesthetic demand for this patient, the 12-month postoperative result showed excellent root coverage and good aesthetic blending of the tissues (Figure 2e). There was little sign of the previous graft with this biomimetic therapy. The maxillary left canine was also successfully treated with a connective tissue graft.
PERIODONTAL REGENERATION
The most challenging aspect of periodontal treatment is regenerating the periodontium – including alveolar bone, periodontal ligament, and cementum – on a previously diseased root surface. Recently the Food and Drug Administration approved a recombinantly engineered growth factor delivered in a synthetic matrix. GEM 21S (Osteohealth) has been shown in a randomized, controlled, double-blind clinical trial to increase clinical attachment and to increase linear radiographic bone growth and percent radiographic bone fill at 6-month post-treatment evaluations.9 GEM 21S combines a potent, periodontally targeted growth factor (rhPDGF-BB) with a synthetic beta tricalcium phosphate (B-TCP) matrix to allow for the regeneration of lost periodontal tissues. Over the past 20 years rhPDGF-BB has been extensively researched for this application and has been shown to be extremely safe. In 2 multicenter studies including a 6-month clinical trial with 120 patients receiving the rhPDGF-BB therapy, there were no serious adverse events related to treatment.10
CASE 2
 |
|
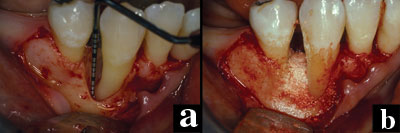
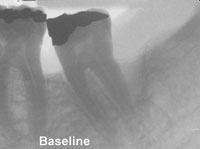
Figures 3a and 3b. A regenerative membrane is difficult to keep covered when placed over a dehisced defect. This site was treated with B-TCP pre-soaked in rhPDGF-BB. |
 |
|
Figure 3c. Radiograph at 36 months shows evidence of bone maturation and stability of the site. |
This patient was diagnosed with aggressive periodontitis and presented with a mandibular right canine with advanced periodontal bone loss. Surgical exposure of the site revealed a 7-mm intrabony defect (Figure 3a). The defect was 1-wall/2-wall and was not well-contained. Traditional approaches with guided tissue regeneration (GTR) would have presented a challenge for managing the soft tissues over the regenerative membrane, and membrane exposure can significantly reduce the success of GTR.
This patient was treated as part of a clinical trial with 1.0 mg/mL of rhPDGF-BB combined with B-TCP. Great care was taken during the surgery to gently elevate the soft tissues and provide a flap with adequate access to remove granulomatous tissue and debride the root surface of calculus. The root surface was conditioned by placing topical tetracycline paste for 4 minutes. Treatment with rhPDGF-BB was applied directly to the cleaned and dried root surface and the defect. The B-TCP was pre-soaked in the rhPDGF-BB and then applied to fill the defect (Figure 3b). The flaps were sutured for primary closure over the defect.
At 12 months, there was radiographic suggestion of bone fill, and re-entry surgery revealed apparent bone fill of the defect and coverage of the previously dehisced buccal root surface. The postoperative radiograph suggested maturation of the treated site, and the 36-month postoperative radiograph evidenced further maturation and stability (Figure 3c).
CASE 3
 |
 |
|
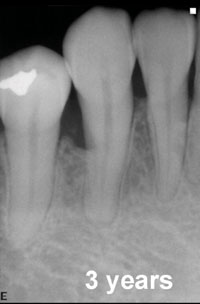
Figure 4a. Radiograph of mandibular second molar with 11-mm pocket on distal-buccal surface. In Figures 4a and 4b, note the bone trabeculation with the inverted radiographic image. |
Figure 4b. Radiographic evidence of bone fill with trabeculation at 3 years following treatment with 0.3 mg/mL rhPDGF-BB and B-TCP (GEM 21S). |
This patient’s mandibular left second molar presented with an 11-mm periodontal pocket on the distal-buccal surface and was treated with GEM 21S (0.3 mg/mL rhPDGF-BB and B-TCP) during the clinical trial (Figure 4a). The preoperative radiograph evidenced a deep vertical defect on the distal surface approaching the apical one third of the distal root. This tooth had a guarded prognosis, but the patient wanted to save it. Evaluation 6 months postoperatively revealed there was a 6-mm reduction in probing depth and a 6-mm gain of clinical attachment. The 3-year postoperative radiograph evidenced bone fill with natural trabeculation and no sign of the original defect (Figure 4b). This tooth now has a good prognosis and has been restored with a full-coverage restoration.
CASE 4
 |
 |
|
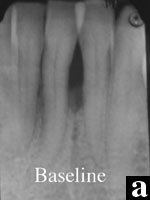
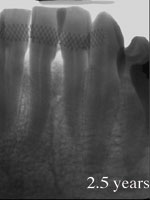
Figures 5a and 5b. The baseline and 2.5-year radiographs of mandibular incisors treated with DFDBA and EMD indicate recalcification of the defect. |
This patient presented with generalized advanced chronic periodontitis (AAP type IV) and had been previously told that her mandibular incisors would require extraction due to advanced periodontal bone loss. The treatment options presented to the patient were either to extract the 4 mandibular incisors and replace them with an implant-supported fixed partial denture, or to utilize periodontal regenerative therapy to maintain the incisors. The patientÃs desire was to save her natural teeth, which determined the treatment plan.
Initial therapy included scaling and root planing, oral hygiene instruction, and occlusal analysis. The occlusal analysis identified the need to control the mobility of the incisors, which was accomplished with occlusal adjustment and provisional splinting with wire mesh and composite resin.
Surgical debridement revealed a 1-wall defect between teeth Nos. 23 and 24. The roots were conditioned sequentially – first with tetracycline prior to root planing and then with neutral pH EDTA prior to applying the EMD gel to the cleaned and dried root surface. The site was treated with a combination therapy: 75% demineralized freeze-dried bone allograft (DFDBA), 25% particulate autogenous bone graft, EMD, and a protective collagen membrane (Bio-Gide [Osteohealth]). The EMD was mixed with the graft and applied over the graft, the membrane, and the surgical wound.
The 4-month postoperative radiograph was suggestive of bone fill, and the 9-month radiograph evidenced recalcification of the treated site. The 30-month postoperative radiograph demonstrates normal trabeculation of the treated site (Figures 5a and 5b). To straighten the mandibular incisors, the patient desires orthodontic treatment, which will be considered after the 36-month evaluation.
CASE 5
 |
 |
|
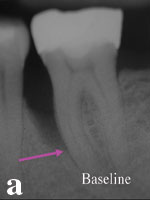
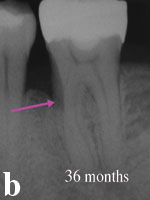
Figures 6a and 6b. An 11-mm defect with improved prognosis 3 years after regenerative treatment. |
This patient presented initially with an 11-mm defect on the mesial surface of his mandibular left first molar. The 3-year radiographic evaluation after combination regenerative periodontal therapy with a bone replacement graft, growth factor, and absorbable membrane reveals a tooth with an improved prognosis. There is significant bone fill of the original defect (Figures 6a and 6b).
CASE 6
 |
 |
 |
 |
 |
|
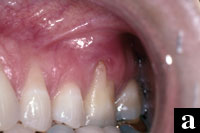
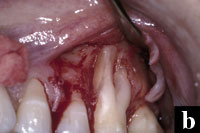
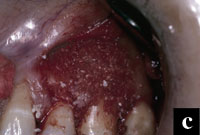
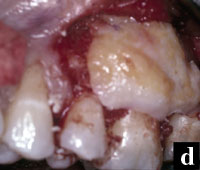
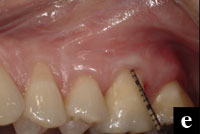
Figures 7a to 7e. A more complex case involves a mucogingival defect, gingival recession, and grade II furcation involvement and is treated with a combination of EMD, DFDBA, a connective tissue graft, and a modified double pedicle technique. At 1 year there is 10 mm of attachment level gain and an adequate zone of attached gingiva. |
More complex problems can be addressed by combining hard- and soft-tissue regenerative procedures. For example, this case presented with a maxillary left first molar with a mucogingival defect, gingival recession, and grade II furcation invasion (Figures 7a to 7e).
The defect site was treated with EMD combined with DFDBA, a connective tissue graft, and a modified double pedicle technique. After flap elevation, degranulation, and root preparation the defect was treated with EMD and then filled with DFDBA hydrated with and pre-soaked in EMD. The connective tissue was then sutured over the bone graft. The overlying flap, which presented with clefting, was repaired with 7-0 polypropylene sutures maintaining the papilla to the original position. A periosteal releasing incision allowed for primary closure over the composite graft. The 12-month clinical evaluation revealed an adequate zone of attached gingiva and approximately 10 mm of attachment gain.
CONCLUSION
The advances that tissue-engineered regenerative medicine is bringing to periodontology are very exciting for researchers, clinicians, and patients. Decades of research has resulted in improved patient care as assessed by clinical and radiographic outcomes. Because of the success of osseointegrated implants, many teeth are being extracted that could be restored to health with current periodontal therapy. It is imperative that clinicians consider all options for therapy, including regenerative periodontics, prior to extracting teeth. Clinicians must communicate these options to their patients.
References
1. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920-926.
2. Lynch SE. The role of growth factors in periodontal repair and regeneration. In: Polson AM, ed. Periodontal Regeneration: Current Status and Directions. Chicago, Ill: Quintessence; 1994:179-198.
3. Giannobile W. Periodontal tissue regeneration by polypeptide growth factors and gene transfer. In: Lynch SE, Genco RJ, Marx RE, eds. Tissue Engineering: Applications in Maxil-lofacial Surgery and Periodontics. Chicago, Ill: Quintessence; 1999.
4. Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-?? (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23:213-225.
5. Langer B, Langer L. Subepithelial connective tissue graft technique for root coverage. J Periodontol. 1985;56:715-720.
6. Wennstrom JL. Mucogingival therapy. Ann Periodontol. 1996;1:671-701.
7. Harris RJ. The connective tissue with partial thickness double pedicle graft: the results of 100 consecutively-treated defects. J Periodontol. 1994;65:448-461.
8. Rasperini G, Silvestri M, Schenk RK, et al. Clinical and histologic evaluation of human gingival recession treated with a subepithelial connective tissue graft and enamel matrix derivative (Emdogain): a case report. Int J Periodontics Restorative Dent. 2000;20:269-275.
9. Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76:2330-2332.
10. Howell TH, Fiorellini JP, Paquette DW, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68:1186-1193.
Dr. Nevins is in the private practice of periodontics and implant dentistry in Boston. He is a Diplomate of the American Board of Periodontology and is an Assistant Clinical Professor in the Department of Periodontology at Harvard School of Dental Medicine. Dr. Nevins graduated from Tufts University School of Dental Medicine and received his certificate for graduate training in periodontology and a master of medical sciences in oral biology at Harvard School of Dental Medicine. He has research interests in clinical applications of tissue engineering for periodontics and implant dentistry. He can be reached at marc_nevins@hms.harvard.edu.
|
Continuing Education Test No. 82.2 |
After reading this article, the individual will learn:
Ô how tissue engineering can improve clinical periodontal outcomes, and
Ô to recognize clinical applications for using amelogenin-like factors and growth factors to enhance the outcome of periodontal therapy.
1. Tissue engineering can achieve regeneration of tissues using ____.
a. scaffolds
b. cells
c. signaling molecules
d. all of the above
2. Periodontal regeneration is documented histologically by observation of which tissues adjacent to the previously diseased root surface?
a. cementum
b. periodontal ligament
c. alveolar bone
d. all of the above
3. B-TCP is used ____.
a. as an anticoagulant
b. as a synthetic matrix in combination with a growth factor
c. as a post-surgical dressing
d. in suture materials
4. rhPDGF-BB is ____.
a. a bone grafting material
b. a periodontally targeted growth factor
c. a solution used to treat root sensitivity
d. an anticoagulant
5. The connective tissue graft for root coverage as presented by Langer and Langer ____.
a. uses a free keratinized soft-tissue graft for root coverage
b. uses free keratinized soft-tissue graft to enhance the zone of attached gingiva
c. uses a connective tissue graft protected by the primary flap to achieve root coverage
d. uses growth factors to enhance the healing
6. Regenerative treatment can be used to treat the following types of defects ___.
a. recession
b. intrabony defects
c. furcation defects
d. all of the above
7. GEM 21S combines the following to treat periodontal defects ____.
a. allogeneic cells and bone
b. beta tricalcium phosphate and tetracycline
c. rhPDGF-BB and allogeneic bone
d. beta tricalcium phosphate and rhPDGF-BB
8. Regenerative procedures utilizing newer technologies such as amelogenin-like factors and growth factors require detailed attention to ____.
a. root debridement
b. flap management
c. postoperative oral hygiene
d. all of the above
To submit Continuing Education answers, download the answer sheet in PDF format (click Download Now button below). Print the answer sheet, identify the article (this one is Test 82.2), place an X in the box corresponding to the answer you believe is correct, and mail to Dentistry Today Department of Continuing Education (complete address is on the answer sheet).