Heart attacks, strokes, and cardiovascular/cerebrovascular diseases are the leading causes of death in the United States, and a number of studies have indicated that obstructive sleep apnea (OSA) is a risk factor for these conditions.1,2 Further, OSA and obesity are correlated, and obesity is linked to increased morbidity and mortality.3 The dental profession has a unique opportunity to play a role in the management of sleep breathing disorders and thereby improve the quality of life for patients seen in the dental office.
The purpose of this article is to review the literature concerning the role for dental practitioners in the recognition and treatment of sleep apnea.
 |
|
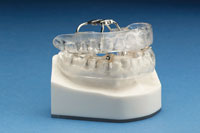
Figure 1. The Klearway appliance allows for incremental, 0.25-mm mandibular movements into a therapeutic position. (Figures 1 and 2 courtesy of Great Lakes Orthodontics.) |
 |
|
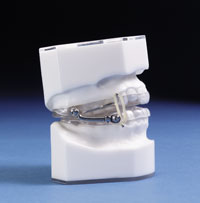
Figure 2. The Herbst Sleep Appliance is retentive and allows for gradual, precise movements of the mandible without the use of shims. |
 |
|
Figure 3. A Herbst-style appliance is pictured above. It is adjustable by adding spacers. (Figure 3 courtesy of Strong Dental.) |
 |
|
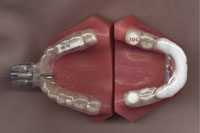
Figure 4. Tap appliance uses a hook on the maxilla to attach to the mandible in order to bring the mandible forward. (Figure 4 courtesy of Johns Dental Laboratories [johnsdental.com].) |
 |
 |
|
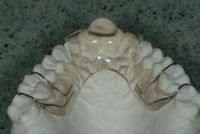
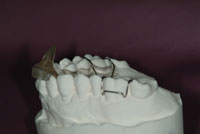
Figures 5 and 6. The Lamberg Appliance reports 95% compliance due to its less bulky design. (Fig-ures 5 and 6 courtesy of Dr. Steve Lamberg.) |
DIAGNOSIS AND TREATMENT OF OSA
Diagnosis and treatment of OSA present a challenge for the clinician. Establishing that a patient has sleep apnea usually requires a polysomnogram (PSG). This test monitors the patient’s sleep patterns for a night to record the number of episodes of apnea, blood oxygen saturation, heart rate, eye movements, breathing, and other variables descriptive of the patient’s sleep. Medical facilities that offer polysomnography are limited in some areas, and scheduling a night to perform the study can be troublesome for patients with busy schedules. The study can only be performed at a sleep study center.
Establishing the appropriate treatment for OSA can be difficult. Many of the therapies are uncomfortable and unappealing to patients. Furthermore, the involvement of several professionals requires interdisciplinary communication. Treatment of OSA can involve a dentist, an oral surgeon, and an otolaryngologist. This can create very real problems for patients. Correcting OSA primarily requires managing the airflow, the domain of the ear-nose-throat physician. But this can mean moving the jaw forward, a dentist’s responsibility, or sometimes surgery of the jaw, an oral surgeon’s responsibility. The impulse can be strong for one practitioner to take the lead over the others. The situation is further complicated if patient compliance is poor.
PATHOGENESIS
Obstructive sleep apnea is characterized by a collapsing of the tongue back onto the pharynx during sleep. Typically, this is because of a large tongue, small air pathway, or otherwise abnormal throat anatomy. This blockage restricts breathing, lowering the concentration of oxygen in the blood until receptors in the carotid sinus are alerted to higher CO2 levels in the body, and air hunger is triggered. The patient then regains consciousness, possibly to a limited extent such that he or she does not recall the episode later. Normal breathing is then restored. When the patient falls into a deep sleep, the tongue will collapse again until another apneic episode occurs.
The apnea-hypopnea index (AHI) measures the number of apnea episodes per hour. An AHI greater than 10 is clinically significant. As will be covered later in the article, waking up every 6 minutes can have many negative consequences, as many important processes occur during sleep. Sleep occurs in roughly 4- to 5-hour cycles of light sleep progressing into deep sleep and then REM sleep; OSA interferes with this rhythm.
Being overweight or obese greatly increases one’s risk for sleep apnea. A number of studies correlate above-average neck circumference to prevalence of sleep apnea.4 Furthermore, evidence suggests that allergies that affect the nasal mucosa and inhibit proper breathing can increase the likelihood of sleep apnea.5
LINKS TO OTHER DISEASES
A growing body of literature suggests a relationship between sleep breathing disorders and cardiovascular/cerebrovascular disorders. Obstructive sleep apnea is associated with atherosclerosis,6 hypertension,7 high blood pressure,8 and type 2 diabetes.9 The mechanisms by which sleep apnea influences these problems are not well understood. OSA causes oxygen deprivation, which is thought to interfere with metabolic processes; a study by Savransky, et al10 showed that intermittent hypoxia, a result of sleep apnea, causes or exacerbates hyperlipidemia and hypercholesterolemia. Leuenberger, et al11 revealed that intermittent hypoxia over even a very short period of time significantly increased sympathetic nervous system activity, caused a stronger chemoreflex response to continuous hypoxia, and elevated blood pressure. This study did not test the effects of periodic hypoxia over extended periods of time, but it is possible that the effect raises blood pressure to a clinically significant extent.
Several studies suggest that those suffering from sleep apnea are at greater risk for involvement in automobile accidents because of daytime sleepiness.12 Given that traffic accidents are a leading cause of death of American adults, it is especially incumbent upon health-care professionals to inform their patients of the harmful effects of sleep apnea.
DIAGNOSIS
Proper diagnosis of OSA requires a polysomnogram. Subjective surveys of daytime wakefulness are also helpful in determining if an individual is suffering from OSA.
Bruxism is associated with sleep apnea and should be the first signal to alert the dentist of the need to pursue this line of questioning. Sleep bruxism is rarely seen independent of sleep apnea, and it has been shown that effective treatment of OSA completely eliminates sleep bruxism.13 Questions about blood pressure and daytime wakefulness will indicate whether a sleep study is in order. Obesity is an important risk factor in developing OSA, and this should be taken into account when referring a patient to a sleep center.
Barsh14 raised the concern about dentists making their own diagnoses of primary snoring and prescribing oral appliance therapy while ignoring the pathophysiology of snoring and sleep apnea. Dental practitioners are in a position to recognize many telltale signs of sleep apnea, such as a small airway and evidence of severe bruxism. However, it should be emphasized that lack of proper diagnosis and lack of consideration for other treatment modalities that fall outside the realm of dentistry can pose a threat to patients’ health. Cooperation with medical professionals in other fields is essential when dealing with this problem.
TREATMENTS
The correlation between being overweight and having an AHI greater than 10 is strong enough that weight management should be recommended to all overweight patients as a means of controlling sleep apnea. Some have suggested that altering sleep positions will influence the frequency of apneas; sleeping on one’s back increases the likelihood that the tongue will block the pharynx. The effectiveness of this behavioral change is usually not enough to reduce the AHI below 10, but may be sufficient in some cases, thereby saving those pa-tients the need for additional therapy.
Continuous Positive Air Pressure
Continuous positive air pressure (CPAP) pumps air at a determined pressure through the nose. The force of the pressure down the air cavity serves as a splint, which keeps the tongue from collapsing back against the pharynx. CPAP is the most prescribed treatment for OSA and is almost always effective, but patient compliance is poor; compliance estimates are 30% to 40%.15 The machine can be large and cumbersome, and its use can have irritating side effects such as nasal congestion and throat dryness. In spite of this, there is no substitute for CPAP in severe cases, as surgical methods to treat the disorder are unpredictable. Compliance with CPAP has been shown to reduce the levels of serum risk factors for cardiovascular/cerebro-vascular disease such as cholesterols and apolipoproteins.16 Patients who are not likely to benefit from oral appliances and are reluctant to accept the CPAP machine should be reminded of the great toll that sleep apnea takes on their health.
Oral Appliances
For patients who cannot tolerate CPAP, an oral appliance may be recommended. These appliances have a number of advantages over CPAP, namely that they are more comfortable, less expensive, and adjustable. The majority of appliances used to correct OSA reposition the mandible; moving the mandible forward also pulls the tongue forward, making it less likely to collapse onto the back of the throat. A few appliances function by retaining the tongue at the front of the mouth using a suction bulb.
While oral appliance therapy to treat OSA is an important treatment option, these appliances are effective in only about half of all cases.17 Enthusiasm for these appliances is due to patients’ preference for them over CPAP, not because of their success rate. When effective, oral appliances re-duce the apnea-hypopnea index below 10 and restore the patient’s quality of life. The remaining cases are too severe, and use of an oral appliance does not reduce the AHI to an extent that eliminates the adverse effects of sleep apnea. In many cases, the reason for the failure of an oral appliance is that OSA is rarely a localized phenomenon within the airway. For example, OSA can be caused by an obstruction in the pharynx.18
A study by Almeida, et al19 indicates that long-term use of a mandibular advancement device can have a clinically significant effect on the patient’s occlusion. In some cases a positive change was observed, although a negative impact was slightly more common. No literature evaluates the degree of mandibular advancement necessary to treat a sleep apnea patient with a particular AHI (Figures 1 to 6).
Surgery
Surgery should be considered as the last resort when treating sleep apnea; it is rare that noninvasive treatments for OSA are ineffective. Surgery is indicated in those cases, or when the patient cannot tolerate CPAP, oral appliance therapy is ineffective, and other treatments such as weight loss have not worked. Evaluation of the airway before surgery requires the cooperation of both an otolaryngologist and oral and maxillofacial surgeon; the potential causes of a blockage are numerous and include the anatomy of the oral and nasal cavities as well as the throat.20
The first widely accepted surgical procedure to treat snoring and sleep apnea was uvulopalatopharyngoplasty (UPPP). This surgery involves removal of the uvula and surrounding soft palate to prevent constriction at the velopharynx. The hypopharynx is often a site of blockage as well, which may explain why UPPP is successful in less than half of all cases. Complications of this surgery are frequent, and include pain, hemorrhaging, and breathing difficulty. Recently, the introduction of surgical lasers has improved UPPP so that complications are fewer and less drastic, but the procedure’s success rate has not improved.20
Another treatment modality is surgery to reposition the hyoid bone. Movement of the hyoid bone affects the tongue’s positioning in the mouth and may alleviate sleep apnea symptoms. By itself, hyoid suspension has a success rate of nearly 60%.21 Several published studies evaluated the combination of hyoid suspension and UPPP, which improved the success rate to approximately 80%.22,23 This finding further supports the idea that airway blockages occur for different reasons, and a combination of treatments may be necessary to achieve an acceptable result.
Removal of tonsils and adenoids can be effective in treating OSA in children because the adenotonsillar tissues are large and have not yet receded. This procedure is not usually indicated in adults with sleep breathing disorders because the adenoids and tonsils are not large enough to block the airway.
Nasal surgery such as turbinectomy and septoplasty may reduce AHI, but rarely below 10. Sleep apnea is rarely caused by problems in the nasal cavity alone, so nasal surgery may be used in combination with other surgical procedures in the throat or mouth, if at all.
DISCUSSION
Sleep apnea is an under-diagnosed disorder, and the consequences it has on general health, including cardiovascular health, should be recognized. Diagnosis and treatment of obstructive sleep apnea will improve the quality of life for patients, and dental professionals should play a role in this aspect of their patients’ health. Given the relative lack of public or professional attention given to sleep apnea, it is appropriate that, when indicated, patients be asked about snoring, daytime wakefulness, and other signs and symptoms of OSA. The presence of severe bruxism is also an important warning sign and should trigger further inquiry on the subject. The use of oral appliances to treat sleep apnea deserves consideration, as they are the best treatment option in approximately half of all cases. Furthermore, correcting sleep apnea offers a unique opportunity to collaborate with practitioners in other fields of medicine.
References
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906-1914.
- Wolf J, Lewicka J, Narkiewicz K. Obstructive sleep apnea: an update on mechanisms and cardiovascular consequences. Nutr Metab Cardiovasc Dis. 2007;17:233-240.
- Grunstein RR, Wilcox I. Sleep-disordered breathing and obesity. Baillière’s Clin Endocrinol Metab. 1994;8:601-628.
- Ward Flemons W, McNicholas WT. Clinical prediction of the sleep apnea syndrome. Sleep Med Rev. 1997;1:19-32.
- Canova CR, Downs SH, Knoblauch A, et al. Increased prevalence of perennial allergic rhinitis in patients with obstructive sleep apnea. Respiration. 2004;71:138-143.
- Drager LF, Bortolotto LA, Figueiredo AC, et al. Effects of CPAP on early signs of atherosclerosis in obstructive sleep apnea [published online ahead of print June 7, 2007]. Am J Respir Crit Care Med. PMID: 17556718.
- Budhiraja R, Sharief I, Quan SF. Sleep disordered breathing and hypertension. J Clin Sleep Med. 2005;1:401-404.
- Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27:934-941.
- Chasens ER. Obstructive sleep apnea, daytime sleepiness, and type 2 diabetes. Diabetes Educ. 2007;33:475-482.
- Savransky V, Nanayakkara A, Li J, et al. Chronic intermittent hypoxia in-duces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290-1297.
- Leuenberger UA, Hogeman CS, Quraishi S, et al. Short-term intermittent hypoxia enhances sympathetic responses to continuous hypoxia in humans [published online ahead of print June 7, 2007]. J Applied Physiol. 2007;103:835-842. PMID:17556498.
- Ellen RL, Marshall SC, Palayew M, et al. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;15:193-200.
- Oksenberg A, Arons E. Sleep bruxism related to obstructive sleep apnea: the effect of continuous positive airway pressure. Sleep Med. 2002;3:513-515.
- Barsh LI. Dentistry’s role in the recognition and treatment of sleep-breathing disorders: the need for cooperation with the medical community. J Calif Dent Assoc. 1998;26:591-598.
- Joo MJ, Herdegen JJ. Sleep apnea in an urban public hospital: assessment of severity and treatment adherence. J Clin Sleep Med. 2007;3:285-288.
- Steiropoulos P, Tsara V, Nena E, et al. Effect of continuous positive airway pressure treatment on serum cardiovascular risk factors in patients with obstructive sleep apnea-hypopnea syndrome [published online ahead of print June 15, 2007]. Chest. 2007;132:843-851. PMID: 17573492.
- Hoffstein V. Review of oral appliances for treatment of sleep-disordered breathing. Sleep Breath. 2007;11:1-22.
- Zonato AI, Bittencourt LR, Martinho FL, et al. Association of systematic head and neck physical examination with severity of obstructive sleep apnea-hypopnea syndrome. Laryngoscope. 2003;113:973-980.
- Almeida FR, Lowe AA, Otsuka R, et al. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 2. Study-model analysis. Am J Orthod Dentofac Orthop. 2006;129:205-213.
- Marshall MW. Surgical options for obstructive sleep apnea. J Calif Dent Assoc. 1998;26:579-590.
- Verse T, Baisch A, Maurer JT, et al. Multilevel surgery for obstructive sleep apnea: short-term results. Otolaryngol Head Neck Surg. 2006;134:571-577.
- Vicente E, Marín JM, Carrizo S, et al. Tongue-base suspension in conjunction with uvulopalatopharyngoplasty for treatment of severe obstructive sleep apnea: long-term follow-up results. Laryngoscope. 2006;116:1223-1227.
- Omur M, Ozturan D, Elez F, et al. Tongue base suspension combined with UPPP in severe OSA patients. Otolaryngol Head Neck Surg. 2005;133:218-223.
Acknowledgment
The author wishes to acknowledge Daniel Shapero for his contribution to this article.
Dr. Singer is in private practice in the areas of Washington, DC, and Alexandria, Va. He is also an assistant clinical professor in the Department of Surgery at George Washington University. He can be reached at (202) 912-9200.