Oral complications of cancer chemotherapy have been recognized for many years. Candidiasis, mucositis, chemo-therapy-induced mucosal ulcerations, and altered salivary function are all well-documented side effects of chemotherapeutic agents.1 In 1983, osteonecrosis associated with head and neck cancer patients who had received radiation to the jaws was first described.2 The pathological changes seen in osteoradionecrosis are believed to be due to a decrease in vascularity resulting in tissue hypoxia secondary to radiation injury of the blood vessels. Osteoradionecrosis of the mandible occurs with a much greater frequency than in the maxilla, most likely due to the solitary mandibular blood supply provided by the inferior alveolar artery. It is well-known that the presence of active carious lesions and/or periodontal disease, as well as post-radiation dental extractions and surgical manipulation, increase the risk for development of osteoradionecrosis.3
A new disease entity occurring in patients who were receiving bisphosphonate therapy was described in late 2003 by Marx (Figure 1). In a letter to the editor of the Journal of Oral and Maxillofacial Surgery, it was noted that 36 patients receiving intravenous (IV) bisphos-phonate (BP) treatment were seen with avascular necrosis of the jaws. Twenty-four patients were on pamidronate (90 mg monthly), and 12 patients were receiving zoledronate (4 mg monthly). Eighteen patients of the 36 patients were on BPs for multiple myeloma, 17 patients were being treated for metastatic breast cancer, and 1 patient was receiving treatment for osteoporosis (OP). All patients presented with necrotic bone in the mandible (80%), maxilla (14%), or both (6%). These lesions were characterized as bisphosphonate-associated osteonecrosis of the jaw (ONJ). In 78% of cases, ONJ was associated with extraction of teeth or surgical manipulation. The remainder of cases appeared spontaneously without any known dental or oral trauma.4
The purpose of this paper is to review the mechanism of action of bisphosphonates, current uses of these drugs, and what is known regarding the pathology of ONJ. The current ONJ literature will be summarized, and recommendations for clinical care based on the analysis of reported cases will be presented.
BISPHOSPHONATE MECHANISM OF ACTION
 |
|
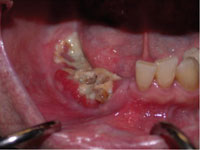
Figure 1. A patient with bisphosphonate-associated osteonecrosis of the mandible. |
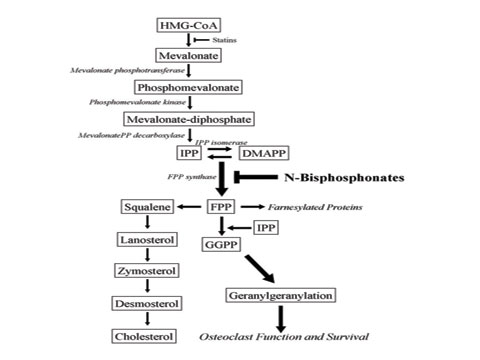 |
| Figure 2. The cholesterol biosynthetic pathway. Inhibition of farnesyl diphosphate synthetase (FPP) by nitrogen-containing bisphosphonates. |
Although BPs were known to be effective inhibitors of osteoclasts, until recently the exact mechanism of action was unclear. New research has elucidated the molecular mechanism of this class of drugs.5 The BP chemical structure is very similar to inorganic phosphate, a regulator of bone metabolism. BPs have a high affinity for divalent ions, particularly calcium, resulting in the in vivo targeting of hydroxyapatite bone mineral surfaces.6 The acidic environment created by the osteoclast within the resorption lacunae can release BPs from the bone surface, where they are internalized through endocytosis.7,8 The first-generation BPs etidronate, tiludronate, and clodronateóare metabolized within the cell to form toxic analogs of ATP, which lead to the initiation of osteoclast apoptosis.9,10 The second and third generation of nitrogen-containing BPs, including pamidronate and zoledronate, act through inhibition of the cholesterol biosynthetic pathway (Figure 2). Several studies have recently shown that farnesyl diphosphate synthetase is inhibited by all nitrogen-containing BPs. This enzyme is critical for the production of cholesterol, isoprenoid lipids, farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP). These isoprenoid lipids are critical for osteoclast functions including but not limited to cell adhesion, formation of a ruffled border, and apoptosis.11,12
PATHOPHYSIOLOGY OF OSTEONECROSIS
Osteonecrosis can affect all skeletal bones, but it is most common in the hip and the mandible.13-19 The pathophysiology of osteo-necrosis appears to be an interruption or compromise of the blood supply as a result of trauma or nontraumatic systemic conditions, including corticosteroid administration, coagulopathies, and alcoholism. While many conditions are associated with osteonecrosis, one of the most significant appears to be hypofibrinolysis.20-23 Glueck and colleagues studied 55 patients with alveolar osteonecrosis and evaluated thrombophilia (increased tendency to form intravascular thrombi), anticardiolipin antibodies (associated with thrombophilia), and hypofibrinolysis (decreased ability to lyse thrombi). Thrombophilia was assessed by activated protein C resistance, while fibrinolysis was quantitated by plasminogen activator inhibitor activity (the major inhibitor of fibrinolysis) and stimulated tissue plasminogen activator (the major stimulator of fibrinolysis). Their data indicated that in 43 of 55 patients (78%), one or more of the tests was positive for thrombophilia or hypofibrinolysis.20 At the present time it is unclear if cancer patients with maxillofacial osteonecrosis have fibrinolytic abnormalities; however, these studies suggest that evaluation of coagulation abnormalities in BP-associated osteonecrosis patients is an important parameter that needs evaluation.
USES OF BISPHOSPHONATES
Bisphosphonates and Hypercalcemia of Malignancy
BPs (inhibitors of osteoclast action) are used in the treatment of postmenopausal and steroid-induced osteoporosis, Paget’s disease of bone, multiple myeloma, hypercalcemia of malignancy, and tumor-associated osteolysis in breast, prostate, lung, and other soft-tissue cancers.24-27 Hypercalcemia of malignancy is a serious complication of cancer that affects 10 to 20% of all cancer patients at some point during the course of their disease.28-30 It can occur in the presence or absence of bone metastases. In patients without skeletal involvement, it occurs most often in squamous cell carcinoma of the lung, head and neck tumors, and in renal cell carcinoma.31 In individuals with bone metastases, hypercalcemia of malignancy occurs in 30 to 80% of multiple myeloma patients, 30 to 65% of pa-tients with metastatic breast cancer, and less than 15% of patients with lung cancer. It occurs when the tumor cells release cytokines and para-thyroid hormone-related proteins as well as other bone-resorbing factors. This resorption of the bone causes an accelerated release of calcium into the circulation, which leads to hypercalcemia, a potentially life-threatening event. Management of this condition includes anti-cancer treatment and IV BPs.31
Bisphosphonates and Breast Cancer
Breast cancer accounts for approximately 30% of all new cancer cases in women. Fifty percent of breast cancer patients develop bone metastases, of which 40 to 70% have complications such as bone pain, pathological fracture, or spinal cord compression.32,33 BPs are currently approved for the treatment and prevention of these skeletal-related events in breast cancer patients with bone metastases. At present, bisphosphonates are approved only for use in patients with bone metastases. However, studies to evaluate their efficacy in the prevention of bone metastases are ongoing.
Bisphosphonates and Multiple Myeloma
Multiple myeloma is the third most common hematologic malignancy in the United States. Myeloma tumor cells have been shown to secrete numerous bone-resorbing cytokines resulting in bone destruction, pathological fractures, hypercalcemia, and pain in a large majority of these patients. Several large placebo-controlled, randomized studies have shown a significant reduction in skeletal-related events (including pathologic fractures, spinal cord compression, radiotherapy or surgery to bone, and hypercalcemia) in multiple myeloma patients treated with bisphosphonates. Additionally, patients on BPs had significantly less myeloma-related bone pain than the placebo group.34,35 New in vitro data show an antiproliferative and apoptotic effect of BPs on multiple myeloma cell lines.36
Case Reports of ONJ
Marx updated his initial report by studying a total of 119 patients with ONJ. Seventy-six of these patients presented to the University of Miami (presumably an additional 40 patients since his original report), and 43 cases were documented by his colleagues at other university centers. Fifty-two percent of patients were under treatment for multiple myeloma, 42% for breast cancer, 3.4% for prostate cancer, and 2.5% for OP. The location of the lesions and comorbidities were similar to the original report.37 An additional 5 cases of suspected BP-associated osteonecrosis of the mandible were reported by Migliorati.38 Three cases developed spontaneously and 2 cases were seen following tooth extraction. No information regarding patient diagnosis or length of BP therapy was presented. Prior to the original report by Marx, Wang et al39 described 3 women receiving chemotherapy for metastatic breast cancer. Two patients developed necrotic bone subsequent to dental manipulation, and 1 patient spontaneously developed necrosis in the maxilla. Histological evaluation revealed necrotic bone without evidence of metastases. Wang, et al postulated that the osteonecrosis was due to the cancer chemotherapy. In a later communication, following their review of other reported cases of BP-associated osteonecrosis, the authors revealed that all 3 patients had been treated with BPs.40
Lugassy, et al41 described 3 patients with multiple myeloma on chronic BP therapy. The first patient had received BPs for 5 years and developed osteonecrosis of the left retromolar area. The osteonecrotic lesion responded to hyperbaric oxygen treatment. The second patient had several occurrences of infected, exposed bone that was ultimately eradicated after sequestrectomy and antibiotic treatment. Histopathologic examination of the surgical specimen revealed infection with Actinomyces. The third patient had developed exposed, infected bone following a mandibular extraction. This patient had been on IV BPs for a 5-year period. Again, histopathology revealed extensive Actinomyces colonization. This patient was successfully treated with large doses of IV penicillin.
Ruggiero et al42 reported on 63 cases from 2 medical centers over a 26-month period. Twenty-eight patients had multiple myeloma, 20 patients were diagnosed with breast cancer, 3 patients had lung cancer, 3 patients had prostate cancer, 1 patient had plasmacytoma, and 1 patient had leukemia. Seven patients were diagnosed with OP and had not received chemotherapy. Thirty-nine patients presented with osteonecrosis of the mandible, and 23 had maxillary involvement. One patient had necrotic lesions in both jaws. Fifty-four of 63 patients had a recent extraction at the necrotic site. The remaining 9 patients had spontaneous bone exposure. Bacterial culture results were reported as normal oral flora. The authors reported that the duration of BP therapy ranged from 6 to 48 months; the treatment time for each individual patient was not noted. Treatment of necrotic lesions ranged from conservative management to resection, with the majority of patients undergoing sequestrectomy. There was no information in this report about oral hygiene in these subjects.
Additional ONJ case series have appeared in the literature since the publication of the initial description of bisphosphonate-associated ONJ.43-45 Migliorati, et al identified 18 patients (17 with bone metastases, one patient with OP) who had ONJ. Ten of the affected patients were under treatment for breast cancer, 3 patients had multiple myeloma, one patient had prostate cancer, one patient had prostate carcinoma/lymphoma, one patient had ovarian cancer, and one patient had ovarian/breast carcinoma. With the exception of the patient with OP who was being treated with oral alendronate, all patients were receiving IV pamidronate or zoledronate with a mean treatment time of 25 months (range of 4 to 41 months). Two patients developed ONJ spontaneously, and the remaining patients developed the disease secondary to dental extractions, infection, or trauma.43 In a report from Belgium, Maerevoet, et al44 reported 9 cases of ONJ in patients receiving zoledronate for skeletal protection. Five patients had breast cancer, and 4 patients had multiple myeloma. Unique to this case series was that all cases of ONJ were spontaneous in that none of the patients had undergone prior dental treatment. The authors reported that at their institution the incidence of ONJ in patients treated with zoledronate was 4.6%.
Durie, et al45 conducted a Web-based survey to evaluate risk factors for ONJ. A total of 1,203 patients (904 with multiple myeloma and 299 with breast cancer) responded to the survey. While this report did not confirm the presence of ONJ, the authors determined that 62 patients with myeloma had ONJ and 54 patients had suspicious findings. In the breast cancer patients, 13 patients were believed to have ONJ and 23 patients had suspicious lesions. Neither corticosteroids nor thalidomide was associated with the development of ONJ; however, 81% of ONJ patients had a history of underlying dental problems. This study reported an ONJ incidence of 10% among patients receiving zoledronate.
| Table 1. Oral Bisphosphonate ONJ Cases.
|
A retrospective chart review of 4,000 patients that received IV BPs at MD Anderson Cancer Center (sponsored by Novartis Pharma-ceuticals, the manufacturer of Aredia [pamidronate disodium] and Zometa [zoledronic acid]) identified 33 cases of ONJ (0.83% overall incidence). Fourteen patients had breast cancer (incidence of 1.2%), and 15 patients were undergoing treatment for multiple myeloma (incidence of 2.8%).46 Similar to the Durie study, none of the cases of ONJ reported were clinically confirmed. Based on the extremely limited data detailed above, the incidence of ONJ in patients receiving IV BPs ranges from 0.83 to 10%. Of note are several recent reports of ONJ in OP patients treated with oral BPs, suggesting that ONJ may be associated with BPs other than zoledronic acid and pamidronate.4,37,42 The incidence of ONJ associated with oral bisphosphonates, however, appears to be extremely low. A recent review of the literature revealed only 13 cases of documented ONJ associated with the use of oral bisphosphonates (Table 1).
LIMITATIONS OF THE CURRENT KNOWLEDGE
While these limited numbers of case reports seem to establish an association between BPs and ONJ, numerous questions regarding the incidence, etiology, pathogenesis, and natural history of this condition need to be addressed. The need for prospective clinical studies to ascertain the risk factors associated with the development of ONJ is obvious. One of the primary factors limiting research on ONJ is the lack of a consistent definition of ONJ, which is necessary before the incidence and prevalence of the disease can be accurately determined. A uniform definition of ONJ is needed to clarify lesions that are “slow healers” (particularly in patients taking multiple anticancer medications) after dental procedures versus true cases of ONJ.
PREVENTION AND MANAGEMENT OF ONJ
Recently, two papers have been published with guidelines related to the prevention and management of ONJ.47,48 One of the papers contains guidelines from the American Academy of Oral Medicine, and the other paper is authored by a group of dentists and oncologists. Both papers present similar approaches to the management of ONJ. It should be noted that the management strategies presented in these papers are not based on clinical studies, but rather on strategies used for patients with osteoradionecrosis.
Guidelines have been developed for 3 distinct groups: 1) patients who will be initiating treatment with BPs; 2) patients who are currently taking BPs; and 3) patients who have developed ONJ.
PREVENTIVE MEASURES PRIOR TO STARTING BISPHOSPHONATE THERAPY
Teeth with a poor prognosis should be extracted, and any other dental surgical procedures should be completed prior to the initiation of BP treatment.
Prior to initiating BP therapy, patients should receive a thorough oral/dental examination, including radiographic evaluation.
The need for good oral hygiene should be stressed.
PREVENTIVE MEASURES FOR PATIENTS CURRENTLY TAKING BISPHOSPHONATES
- While taking BPs, it is recommended that patients should have dental recall visits every 3 to 6 months.
- Routine dental cleaning should be performed with care taken to prevent soft-tissue injury.
- Removable dentures should be checked for their potential to induce trauma.
- Endodontic therapy is preferred over tooth extraction.
- Elective therapy such as implant treatment should be avoided in patients on IV bisphosphonates.
- If surgery is necessary, antibiotics should be considered both presurgically and postsurgically. It has been suggested that antibiotics be given prior to invasive dental procedures and continued for at least 10 days after a dental procedure. Amoxicillin has been recommended as a good first choice, with azithromycin or quinolones as second choices.
- Primary closure of all oral wounds including extraction sites should be attempted.
- Treatment of patients with osteonecrosis of the jaw
- Because of impaired wound healing in patients with ONJ, a nonsurgical approach to the management of these lesions is recommended.
- Only minimal bony debridement of the ONJ lesion should be performed to remove sharp edges.
- Removable appliances should be constructed to protect the ONJ lesion from further trauma.
- Chlorhexidine mouth-rinses (0.12%) are recommended.
The use of antibiotics to treat ONJ lesions has had equivocal results. Antibiotic use should be based on clinical judgment, although choice of an antibiotic should be determined by culture and sensitivity testing of the lesion, if possible. It has been suggested that a combination of amoxicillin and metronidazole may be helpful.47,48
| Table 2. Hyperbaric Therapy for ONJ at the Duke Center for Hyperbaric Medicine and Environmental Physiology (CHMEP).
|
During examination and culture of ONJ lesions, the possibility of fungal infection should be considered. In lesions where cultures have demonstrated the presence of a fungal infection, patients should be placed on nystatin oral rinses or mycostatin troches.
Hyperbaric oxygen (HBO) therapy has been employed to prevent and treat osteoradionecrosis in the maxillofacial bones. The data regarding the use of HBO therapy in ONJ patients has been controversial. Initial reports indicated that HBO was not effective in the treatment of ONJ. However, the treatment protocols as well as the criteria for success were not reported, or HBO protocols for the treatment of osteoradionecrosis (20 dives at 2.0 atm) were used. A recent study, however, from investigators at Duke University Medical Center demonstrated that HBO may be effective in resolving ONJ lesions (Table 2). In that report, they used an intensive treatment protocol that consisted of between 40 and 120 dives and achieved complete resolution of ONJ in 67% of cases.49 This preliminary study suggests that further research is needed to determine optimal therapy for patients with ONJ and underscores the need for a prospective clinical trial to evaluate the efficacy of HBO in the treatment of BP-associated ONJ.
SHOULD BISPHOSPHONATE THERAPY BE STOPPED IN PATIENTS WHO DEVELOP ONJ?
There is no clear evidence that suggests that stopping BP therapy has any effect on the healing of an ONJ lesion. Since BPs have an extremely long half-life in the bone, it would seem that stopping BP therapy would not have a dramatic effect. The decision to stop BPs should be made by the patient’s physician and is based on the risk/benefit ratio determined for each case.
SUMMARY
ONJ appears to be associated with BPs; however, the pathophysiology, incidence, and co-morbidities require further investigation. The major risk factors identified to date appear to be cancer (or chemotherapy for cancer) and dental procedures or oral trauma. A clear definition of ONJ is critical to understanding this disease entity. Although recommendations regarding the prevention and management of ONJ exist, clinical studies are needed to establish more definitive guidelines for the management of ONJ. The use of intensive hyperbaric oxygen therapy may be beneficial to patients with ONJ.
Dr. Landesberg is associate professor in the Division of Oral and Maxillofacial Surgery in the Columbia University College of Dental Medicine and interim director of the Oral and Maxillofacial Surgery outpatient clinic. She received her DMD from the University of Connecticut, School of Dental Medicine, and her residency training in oral and maxillofacial surgery at Strong Memorial Hospital/University of Rochester. Dr. Landesberg has a PhD in microbiology/immunology from the University of Rochester and completed a post-doctoral research fellowship in the Department of Orthopedics at the University of Rochester. She maintains a private practice and is an attending surgeon in oral and maxillofacial surgery at the Columbia University Medical Center/ New York Presbyterian Hospital. Her areas of research include the elucidation of the molecular basis of temporomandibular joint dysfunction/arthritis, the mechanisms involved in the pathogensis of osteonecrosis of the bone, and engineering new delivery systems for the regeneration of craniofacial bone. She can be reached at (212) 305-7626 or RL351@columbia.edu.
Dr. Wilson is chief resident in oral and maxillofacial surgery at Columbia University College of Dental Medicine and the Columbia University Medical Center/New York Presbyterian Hospital. He received his DDS from Columbia University College of Dental Medicine and his MD from Columbia College of Physicians and Surgeons. He can be reached at (212) 305-7626 or tbw4@columbia.edu.
Dr. Grbic is professor and director of the Division of Oral Biology and the Center for Clinical Research in Dentistry at the Columbia University College of Dental Medicine. He received his DMD from the Fairleigh Dickinson School of Dentistry and his certificate in periodontology and a MMSc in oral biology from the Harvard School of Dental Medicine. His research interests include the diagnosis and pharmacotherapeutic management of periodontal diseases, the relationship between periodontal disease and systemic diseases, and the design and conduct of clinical trials. Dr. Grbic maintains a private practice in periodontics in New York City. He can be reached at (212) 305-4640 or jtg2@columbia.edu.
Disclosure: Drs. Landesberg and Grbic are consultants for the Novartis Pharmaceuticals Corporation.
References