It is interesting to consider that dental education relies upon the concepts of classical Newtonian mechanics. Dentists are taught how mechanical work is produced from the force of a drill on a tooth, and that the heat that results (via friction) is a byproduct of the drill’s action on the tooth.1,2 Dentists are generally familiar with how things move, the forces that move them, and the thermodynamic consequences of these forces. These are accurate explanations for everyday clinical experiences in dentistry.3
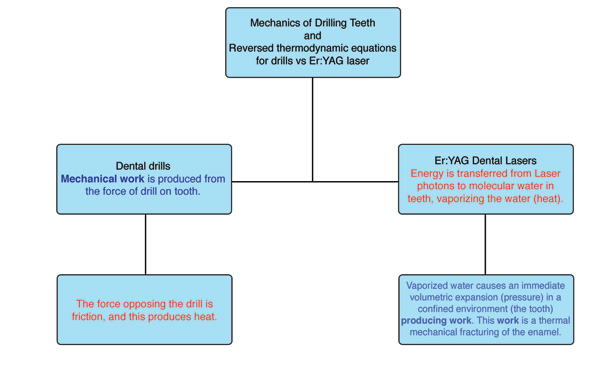 |
| Figure 1. Difference between the mechanics and thermodynamics of drills and Er:YAG lasers. |
When performing a procedure with an Er:YAG laser and a contact sapphire tip with the intention of cutting calcified biologic tissues (teeth and bone), a clinician has entered the extraordinary quantum world of very small distances between atoms and molecules. Er:YAG laser photons (traveling at 186,000 miles/sec) uniquely target the chromophore of molecular water within calcified tissue, but not the calcified structure itself.5 Photon absorption by selected molecular chromophores in a biologic tissue (in this case water), with the concomitant quantum interactions, is referred to as the science of photobiology and is a discipline that does not behave in a classical Newtonian manner. Photobiology is largely unfamiliar to dentists and focuses on the interactions of the nearly instantaneous transfer of quantized packets of energy (laser photons) to selected chromophores (absorptive molecules) in vital tissues.5
 |
|
Figure 2. Tetrahedral geometry of a water molecule. |
 |
| Figure 3. Hydrogen bonds between water molecules. |
 |
| Figure 4. Water freely entering the ablation area. |
 |
| Figure 5. Tip embedded in ablation crater without room for removal of ablation products or water cooling spray. |
 |
| Figure 6. Thermal animation of continually firing contact tip embedded in tissue resulting in a quantum heat trap. |
 |
| Figure 7. Up-and-down motion used for a retrograde endodontic preparation. |
 |
| Figure 8. Back off from the ablation crater, allow the crater to cool, flush, and start again. |
 |
| Figure 9. Alveoloplasty at an angle of 30° with copious water spray on the ablation site. |
 |
| Figure 10. Up-and-down handpiece motion. |
Photobiology and its quantum consequences rely upon a set of laws with which dental practitioners need to be familiar, and hence a dentist using a laser in clinical practice should have a perfunctory understanding of governing principles when cutting (ablating) calcified tissues (teeth and vital bone) with Er:YAG lasers. As noted, the mechanics, interactions, and logic of cutting teeth and bone with lasers is fundamentally different than that of using a conventional dental drill. With dental Er:YAG laser devices and contact tips, the concept that exerting more force—or pushing the tip harder against the tooth—will result in faster cutting does not apply. With Er:YAG lasers and contact tips, a dentist must use an up-and-down motion and back off from a growing ablation crater (laser-drilled hole) to accomplish safe and predictable cuts in calcified tissues. This is related to certain quantum interactions. Only with an understanding of these quantum interactions can a dentist be effective and make correct clinical decisions.
ER:YAG LASERS AND WATER ABSORPTION
Atoms in a molecule are never at rest, and for each type of molecule there are standard vibration modes. For water molecules, there are 3 normal modes of vibration, referred to as symmetric stretching, bending, and assymmetric stretching. These vibration modes depend entirely on the atomic geometry of the covalent bonds (–OH) in the water molecules and carry specific vibrational frequencies.6 The Er:YAG laser at a wavelength of 2.94 µm has the highest specificity for water absorption of all the mid infrared lasers.7 This is because one of the specific molecular vibration modes in the water molecule generates a frequency that corresponds directly to the mid infrared wavelength of of the Er:YAG laser (2.94 µm).6 It is for this reason that the absorption peak in water for Er:YAG energy is so high (Figures 2 and 3).
Importance of hydrogen bonds
The covalent (–OH) bonds in water molecules will bend and stretch depending on the state of the hydrogen bonds between water molecules. Hydrogen bonds are weak forces that attract water molecules to each other and hence give water its property of cohesion. These facts are important because, as noted above, it is the vibrational modes in water molecules that are directly targeted by 2.94-µm Er:YAG optical energy. Further, these vibrational modes are directly influenced by changes in the hydrogen bonds between the water molecules. For example, if the temperature of water is raised significantly, the hydrogen bonds attracting the water molecules to each other will weaken (and conversely the covalent –OH bonds will strengthen), changing the atomic geometry of the water molecules. Any geometric change in the molecule will then negatively alter the absorption peak for water (because the vibrational frequencies are also altered), and this will make Er:YAG lasers less efficient.6
NONCONTACT HARD-TISSUE ABLATION WITH ER:YAG LASERS
One review of the literature indicated that a major clinical advantage of an Er:YAG laser for dental applications is its ability to ablate teeth and bone with minimal thermal damage.8 The photobiology of the Er:YAG-enamel interaction has been defined.9-11 Water molecules within the prismatic enamel layer of a tooth make up only 4% of its chemical composition but represent 11% of enamel’s total volume. As the Er:YAG laser energy interacts with the enamel matrix, water absorbs the laser energy and rapid vaporization occurs. As this photothermal reaction takes place, the steam generated within the enamel is associated with a volumetric expansion and greatly increased pressure within the enamel matrix. This in turn produces microevaporative explosions that result in a thermally driven, mechanical ablation of the tooth structure.11
(1) As water absorbs more of the incident energy of an Er:YAG laser and the temperature of the water increases, the length and strength of the OH bond in the water molecule changes because of the large increase in kinetic energy.
(2) When this important phenomenon occurs, the absorption peak for the water molecule shifts to wavelengths that are significantly shorter than 2.94 µm.
(3) The peak water absorption coefficient for Er:YAG photons at high laser intensities (more energy and heat) drops to the equivalent of only 25% of the water molecules’ original absorption ability at room temperature. This can occur almost instantaneously as a lazed area heats up. This negative shift in the absorption peak for water greatly diminishes the ability of the beam to perform thermal mechanical ablation of tissues.
These findings describing Er:YAG ablation dynamics have profound clinical implications for the interaction of these lasers with mineralized dental tissues, particularly with the use of contact energy delivery tips. For example, as the Shori conclusions are directly applicable to nonmineralized (soft) tissue, energy needed for hard-tissue ablation will be greater, meaning the heat produced by the laser will be greater, and residual heat from the ablation will remain in the mineralized tooth or bone longer than what would occur in soft tissue. If a clinician is using a small-diameter contact tip in an incorrect manner and happens to embed the tip within calcified tissue (tooth or bone), a second negative quantum occurrence will transpire that will fuel a marked heat increase in the system and greatly inhibit further ablation. This second occurrence is based on the Pauli exclusion principle of quantum mechanics (described below) and should be avoided.3,14
CONTACT CUTTING OF CALCIFIED TISSUES WITH ER:YAG LASERS AND THE PAULI EXCLUSION PRINCIPLE
It has been shown that an adequate supply of water directed to the target tissue and the explosive ejection of boiling products of ablation are of paramount importance for efficient Er:YAG ablation. If properly performed, overheating, cracking, and melting of calcified biologic structures during the ablation (which can occur with either contact or noncontact energy delivery systems) will not occur. However, as ablation (laser drilling) occurs in these tissues and a crater forms, the quantum rules that have been described begin to change and need to be considered.
THE QUANTUM HEAT TRAP AND THE ER:YAG LASER WITH A CONTACT TIP
The quantum heat trap occurs as a consequence of 2 phenomena. The first is the clinical manifestation of the Pauli exclusion principle. This occurs because the addition of exogenous water to the ablation site is completely blocked. The heat from the laser is trapped as the boiling material ejected from the ablation area is prevented from leaving the ablation crater. As previously described, this occurs because the physical volume of the solid sapphire contact tip is filling the crater. The second is the continuous deposition and “stacking” of pulses from the laser into the apex of the ablation crater, triggering a negative absorption shift for water to lower wavelengths. This is represented in Figure 6.
METHOD TO AVOID THE QUANTUM HEAT TRAP
A practitioner using an erbium dental laser on hard tissue should never keep the contact energy delivery tip in one place on the tooth for more than 2 to 4 seconds at a time. Within the area the laser will cut, the clinician should at all times combine an up-and-down motion with a side-to-side motion. Use of these motions (instead of pushing the tip into the solid tissue like a bur) will prevent negative quantum interactions from occurring. These recommendations will effectively allow the following:
(1) the boiling products of ablation to explosively exit from a growing crater, and (2) allow more water into the crater to act as a heat sink and flushing mechanism (Figures 7 and 8).
These results were essentially duplicated by Aoki, et al26 observing substantial equivalence in bony cuts, morphology, and chemical composition of bone when the cuts were made with a contact Er:YAG laser or a rotating bur.
CONCLUSIONS
The following conclusions can be drawn:
- The conventional New-tonian mechanics associated with drilling hard dental tissues, including bone, must be superseded with nontraditional quantum mechanics when drilling calcified structures with a Er:YAG laser in the contact mode.
- The target chromophore of water undergoes significant changes in its ability to further absorb Er:YAG laser radiation if there is a large temperature change in the system being irradiated.
- By changing the approach and protocol when cutting with Er:YAG lasers and contact tips, the quantum heat trap leading to “stall out” can be avoided.
For the Er:YAG dental laser systems, a practitioner must make maximum use of the water spray focused on the ablation area. All available data would preclude the use of Er:YAG lasers for “closed” or “flapless” surgical procedures for many of the reasons discussed above. If these systems are used with side-to-side and up-and-down motions as well as copious irrigation at the site of ablation, it will promote the most efficient and least damaging ablation of tooth and bone.
References
1. Baum L. Textbook of Operative Dentistry. 2nd ed. Philadelphia, Pa: WB Saunders; 1985.
2. Stannard JG. Materials in Dentistry. 2nd ed. Hanover, Mass: Denali Publishing; 1988.
3. Serway R. Physics for Scientists and Engineers With Modern Physics. 2nd ed. Philadelphia, Pa: Saunders College Publishing; 1986:93-95, 991.
4. Hewitt PG. Conceptual Physics. 9th ed. San Francisco, Calif: Addison Wesley Pub Co; 2001:496-635.
5. Niemz MH. Laser-Tissue Interactions: Fundamentals and Applications. 2nd rev ed. Berlin, Germany: Springer-Verlag; 2002.
6. Welch AJ, Van Gemert MJC. Optical-Thermal Response of Laser-Irradiated Tissue. New York, NY: Plenum Pub Corp; 1995:865-902.
7. Hale GM, Querry MR. Optical constants of water in the 200 nm to 200 µm wavelength region. Appl Opt. 1973;12:555-563.
8. Bornstein ES, Lomke MA. The safety and effectiveness of dental Er:YAG lasers: a literature review with specific reference to bone. Dent Today. Oct 2003;22:129-133.
9. Walsh JT Jr, Flotte TJ, Deutsch TF. Er:YAG laser ablation of tissue: effect of pulse duration and tissue type on thermal damage. Lasers Surg Med. 1989;9:314-326.
10. Walsh JT Jr, Deutsch TF. Er:YAG ablation of tissue: measurement of ablation rates. Lasers Surg Med. 1989;9:327-337.
11. Moshonov J, Stabholz A, Leopold Y, et al. Lasers in dentistry. Part B—Interaction with biological tissues and the effect on the soft tissues of the oral cavity, the hard tissues of the tooth and the dental pulp [in Hebrew]. Refuat Hapeh Vehashinayim. 2001;18(3-4):21-28, 107-108.
12. Wigdor HA, Visuri SR, Walsh JT Jr. Effect of water on dental material ablation of the Er:YAG laser. Proc SPIE. 1994;2128:267-272.
13. Visuri SR, Walsh JT, Wigdor HA. Erbium laser ablation of hard tissue: Control of the thermal load. Proc SPIE. 1994;2134A;130-133.
14. Hey T. The New Quantum Universe. 2nd ed. Cambridge, England: Cambridge University Press; 2003:1-15, 107-111.
15. Nahen K, Vogel A. Shielding by the ablation plume during Er:YAG laser ablation. Proc SPIE. 2001;4257:282-297.
16. Hibst R, Keller U. Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate. Lasers Surg Med. 1989;9:338-344.
17. Walsh JT, Deutsch TF. Measurement of Er:YAG laser ablation plume dynamics. Appl Phys B. 1991;52:217-224.
18. Venugopalan V, Nishioka NS, Mikic BB. The thermodynamic response of soft biological tissues to pulsed infrared-laser irradiation [published correction appears in Biophys J. 1996;71:3530]. Biophys J. 1996;70:2981-2993.
19. Majaron B, Plestenjak P, Lukac M. Thermo-mechanical laser ablation of soft biological tissue: modeling the micro-explosions. Appl Phys B. 1999;69:71-80.
20. Truong MT, Majaron B, Pandoh NS, et al. Erbium:YAG laser contouring of the nasal dorsum: a preliminary investigation. Proc SPIE. 2001;4244:113-120.
21. Vodopyanov KL. Bleaching of water by intense light at the maximum of the lambda equals 3 µm absorption band. Sov Phys JETP. 1990;70:114-121.
22. Vodopyanov KL. Saturation studies of H2O and HDO near 3400 cm-1 using intense picosecond laser pulses. J Chem Phys. 1991;94:5389-5393.
23. Shori RK, Walston AA, Stafsudd OM, et al. Quantification and modeling of the dynamic changes in the absorption coefficient of water at lambda equals 2.94 µm. IEEE Journal of Selected Topics in Quantum Electronics. 2001;7:959-970.
24. Majaron B, Lukac M, Sustercic D, et al. Threshold and efficiency analysis in Er:YAG laser ablation of hard dental tissue. Proc SPIE. 1996;2922:233-242.
25. Sasaki KM, Aoki A, Ichinose S, et al. Scanning electron microscopy and Fourier transformed infrared spectroscopy analysis of bone removal using Er:YAG and CO2 Lasers. J Periodontol. 2002;73:643-652.
26. Aoki A, Yoshino T, Akiyama F, et al. Comparative study of Er:YAG laser and rotating bur for bone ablation. Int Congr Ser. 2003;1248:389-391.
Dr. Bornstein graduated from Tufts University School of Dental Medicine in 1992 and the Maimonides Medical Center General Practice Residency program in Brooklyn, NY, in 1993. He has been using lasers in his dental practice since 1995, and practices general, implant, and laser dentistry in Natick, Mass. Dr. Bornstein can be reached at drericdmd@mindspring.com.
Disclosure: Dr. Bornstein is the chief science officer for NOMIR Medical Technologies, a compa











