INTRODUCTION
With the significant number of endosseous root form implants placed each year (approximately 3 million in the United States), there continues to be a direct proportional increase in peri-implantitis cases. The number varies per study, with reports of 26% in patients.1 Likewise, there are multiple opinions on the etiology of implant disease that vary from the always mentioned biofilm-induced to restorative-related issues to today’s theories, including the theory of titanium implant corrosion.
The American Academy of Periodontology now includes a classification of implant disease. It suggests that the definition is a plaque-associated pathologic condition with progressive loss of supporting bone as defined by radiographic data. The progress is not linear and is considered an acceleration beyond what is noted with periodontitis progression.2
The etiology is critical, and the reversal of peri-implantitis can be related somewhat to that which was responsible for the initial inflammation. There exist many prescribed techniques for managing peri-implantitis that range from monitoring (watch and wait) to options of explanation and replacement with a new implant fixture. Decisions on therapy directions can be based on the severity of the peri-implantitis.
Most surgical procedures utilized in managing peri-implantitis focus on 3 major objectives:
1. Degranulation. It is essential that granulation tissue resulting from inflammation be removed from the surgical site. The removal of granulation helps reduce LPS and pro-inflammatory cytokines. The process also allows for better visualization of any local factors, such as resin cement, or prosthetic issues, such as implant fractures. It also allows for the determination of the extent of osseous resorption and the characteristics of the boney topography. Two-dimensional radiographs are generally not accurate, especially in a facial/buccal to lingual/palatal view, to characterize boney walls. Degranulation can be a challenge in peri-implant sites due to irregular bony topography and limited visualization, resulting in compromised access with conventional instrumentation. Moreover, the instrumentation utilized can have adverse effects on the implant surface when devices such as power-driven ultrasonic instruments come in contact with titanium surfaces.
2. Decontamination of implant surfaces. References to enhancing the bone-implant contact suggest that the exposed implant surface requires detoxification.3 This can consist of the removal of cement and/or biofilm associated with peri-implant disease. Currently, mechanical and chemical decontamination methods are most popular. However, studies have shown that mechanical instrumentation can scratch and pit the implant surface. Chemical decontamination, such as use of citric acid, EDTA, tetracycline, and phosphoric acid, has been recommended, in addition to mechanical methods, to increase effectiveness. Some chemical interventions can create collateral damage to healthy tissue, resulting in delayed wound healing. There is increasing concern about the implant surface itself in long-standing peri-implant surfaces exposed to biofilm. Metallosis can result from corrosive damage to the titanium dioxide (TiO2) layer. When this layer is removed, it can expose the non-oxidized portion of the implant surface, resulting in a release of titanium particles and ions. This corrosive process has been found on diseased dental implants.4
3. Decortication. Studies demonstrate that limited removal of the cortical plate allows for exposure of the cancellous bone in both periodontal and peri-implant lesions, releasing bone morphogenic proteins necessary for regenerative wound healing.5 Decortication can be implemented with a variety of devices, including handpiece drills. On occasion, traditional dental handpieces cannot perform decortication properly due to an inability to access the tortuous defects. Laser tips (with diameters of 0.5 to 0.6 mm) can gain access to deep bony defects where no other instruments can and not damage implant surfaces.
The utilization of dental lasers to manage peri-implantitis is not new. Appreciating the physics of laser light energy and how different wavelengths have an affinity for different targets (chromophores) is critical to having an effective dental laser action. When lasers interact with a target, the beam is either reflected; scattered; transmitted through; or, preferably, absorbed.
Incorporating dental lasers as monotherapy has demonstrated limited value in managing peri-implantitis over conventional flap and resection or augmentation methodologies. However, utilizing lasers to assist/augment peri-implantitis management has rendered favorable results.6-8 Studies have utilized erbium lasers such as Er,Cr:YSGG or Er:YAG for decontamination, degranulation, and decortication. The erbium wavelength demonstrates an enhanced ability to decontaminate titanium surfaces and have increased success when respective settings and techniques are in defined protocols.
CASE REPORTS
Case 1: Managing Early Peri-implantitis
A 38-year-old female patient presented with a chief concern of “receding gum around my implant.” The medical history was uneventful, and she did not have a history of nicotine use. Six years prior, the maxillary left central had fractured after endodontic therapy. The tooth was extracted using a minimally invasive technique, and an immediate implant was placed with freeze-dried mineralized cortical bone and completed with a final restoration.
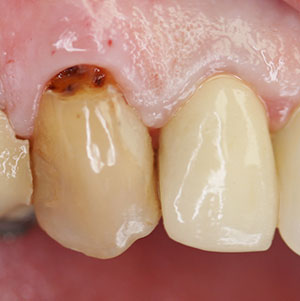
At the current examination, the patient presented with bleeding on probing, generalized suppuration, and recession (Figure 1).
Figure 1. The patient presents on examination with significant recession, bleeding, and suppuration on probing around an implant in the maxillary left central area.
The radiograph analysis demonstrated crestal bone loss (Figure 2).
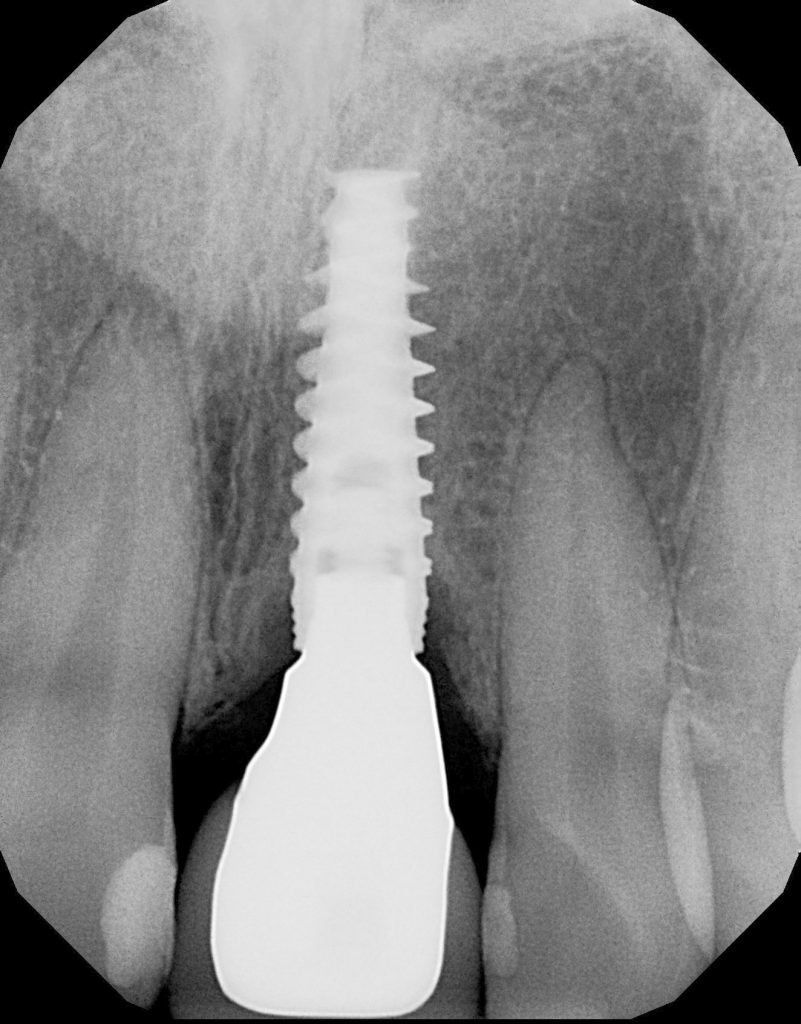
Figure 2. A radiograph demonstrates bony resorption around the crestal bone with the appearance of angular defects.
The diagnosis was early peri-implantitis with submarginal cement and minimal attached gingivae as possible contributing factors.
The patient agreed to an implant repair procedure utilizing an Er,Cr:YSGG all-tissue laser (Waterlase Express [BIOLASE]). The implant crown and abutment were removed to allow better access to the implant defect. The laser was used to de-epithelialize the marginal gingival collar to slow future epithelial migration into the wound.
Vertical incisions were created to expose the surgical site, and a full-thickness flap was reflected. Granulation tissue was effectively removed with the Er,Cr:YSGG laser using a combination of end- and radial-firing laser tips (Figure 3).
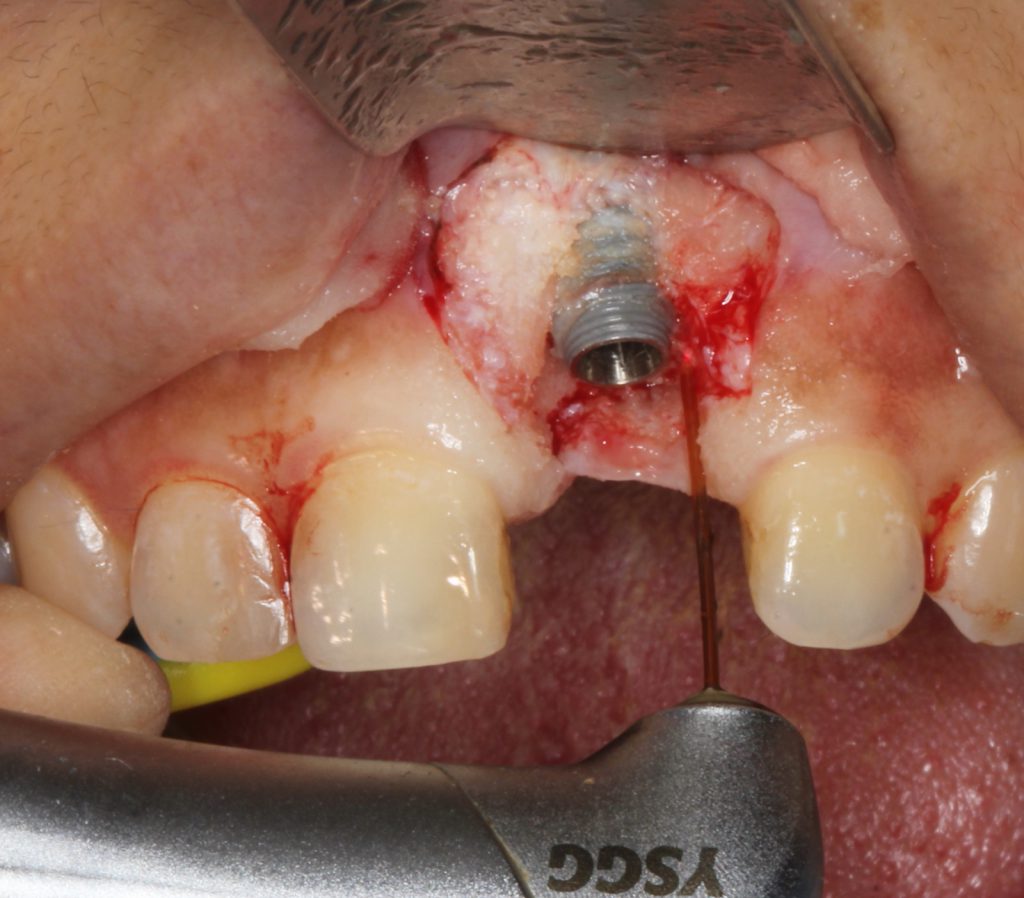
Figure 3. Vertical incisions were made for access, sparing interdental papillae, and the removal of granulation was performed with an Er,CR:YSGG all-tissue laser (Waterlase Express [BIOLASE]) utilizing a radial-firing tip.
The exposed titanium implant surface was decontaminated with the erbium laser using a unique sapphire side-firing tip (Figure 4).
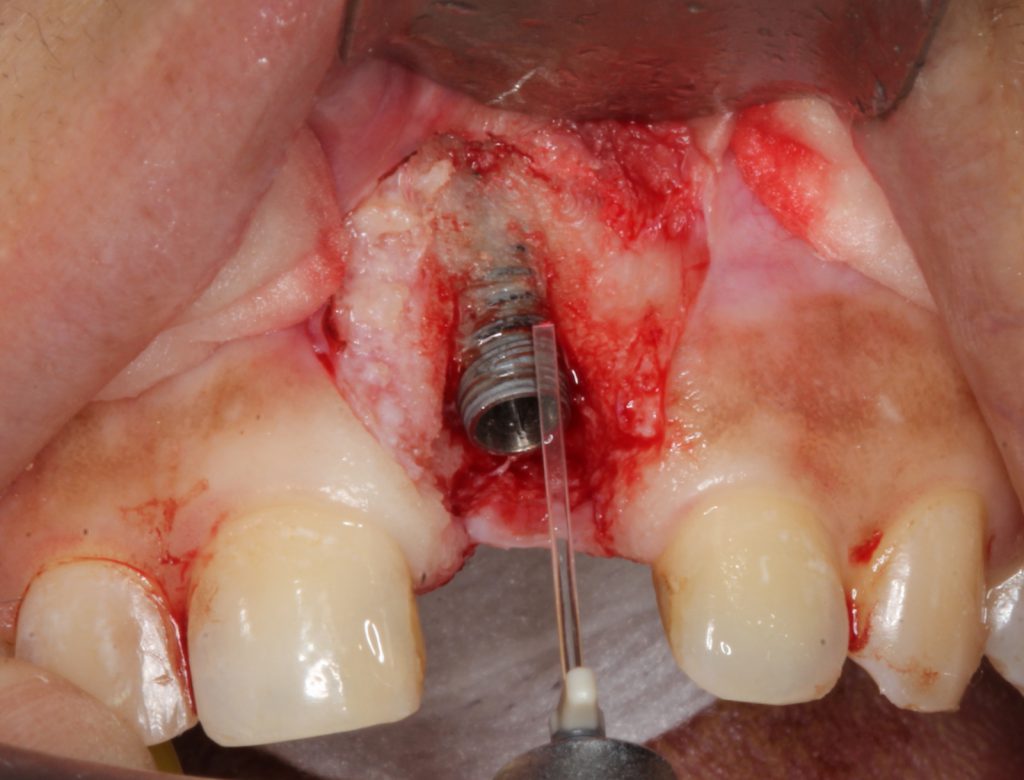
Figure 4. To detoxify/decontaminate the titanium surface, a unique sapphire right angle tip was aimed into the pitch of the threads and cleaned the surface for future re-osteointegration.
The tip allowed energy to leave it in a perpendicular manner to achieve decontamination into the inner aspects of the pitch of the threads. This is especially critical for implants that have angular defects, which make access difficult. Adjacent alveolar bone, including angular defects, was decorticated using an end-firing, zirconium, 0.6-mm laser tip (Figure 5).
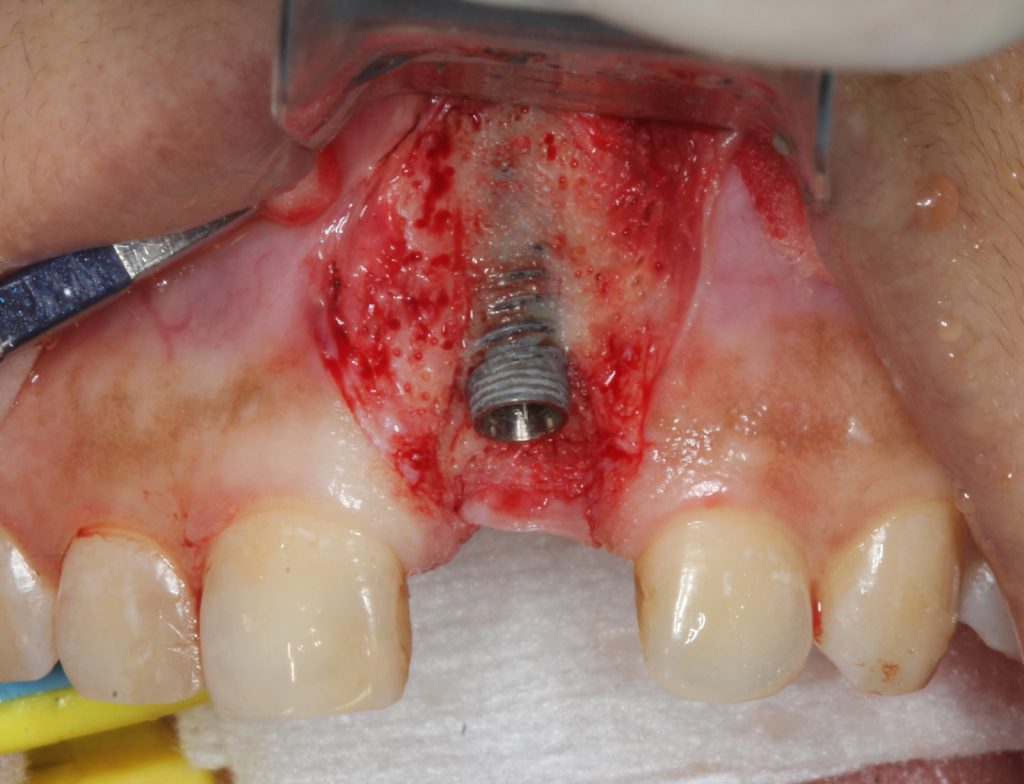
Figure 5. An end-firing tip with the Er,CR:YSGG laser photo acoustics decorticated the bone with the potential to release bone morphogenic protein to enhance osseous regeneration.
A biologic of enamel matrix protein was applied to the site, and freeze-dried mineralized bone was placed with a membrane (Figure 6). A connective tissue graft was harvested from the palate and placed on the site, and the flap was coronally advanced over the entire surgical site (Figures 7 and 8). The existing abutment and crown were reattached to the implant immediately. Photo biomodulation (PBM) for wound healing was directed toward the surgical site with a 940-μm diode laser (Epic X [BIOLASE]).
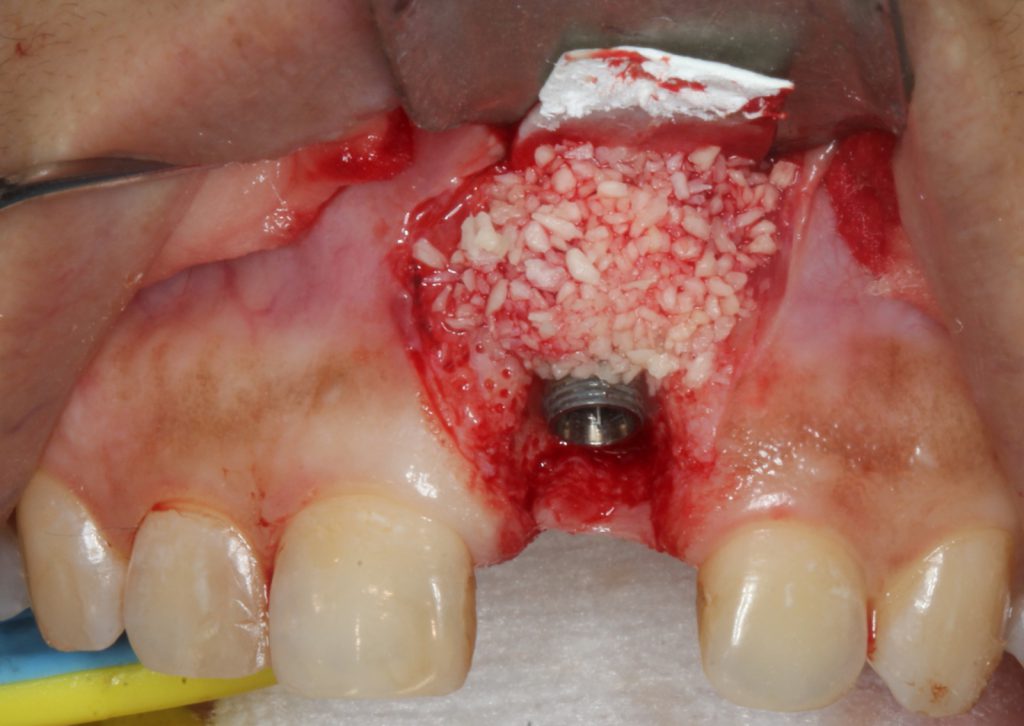
Figure 6. Due to angular bony walls, the defects were augmented with freeze-dried bone allograft and a subsequent membrane.
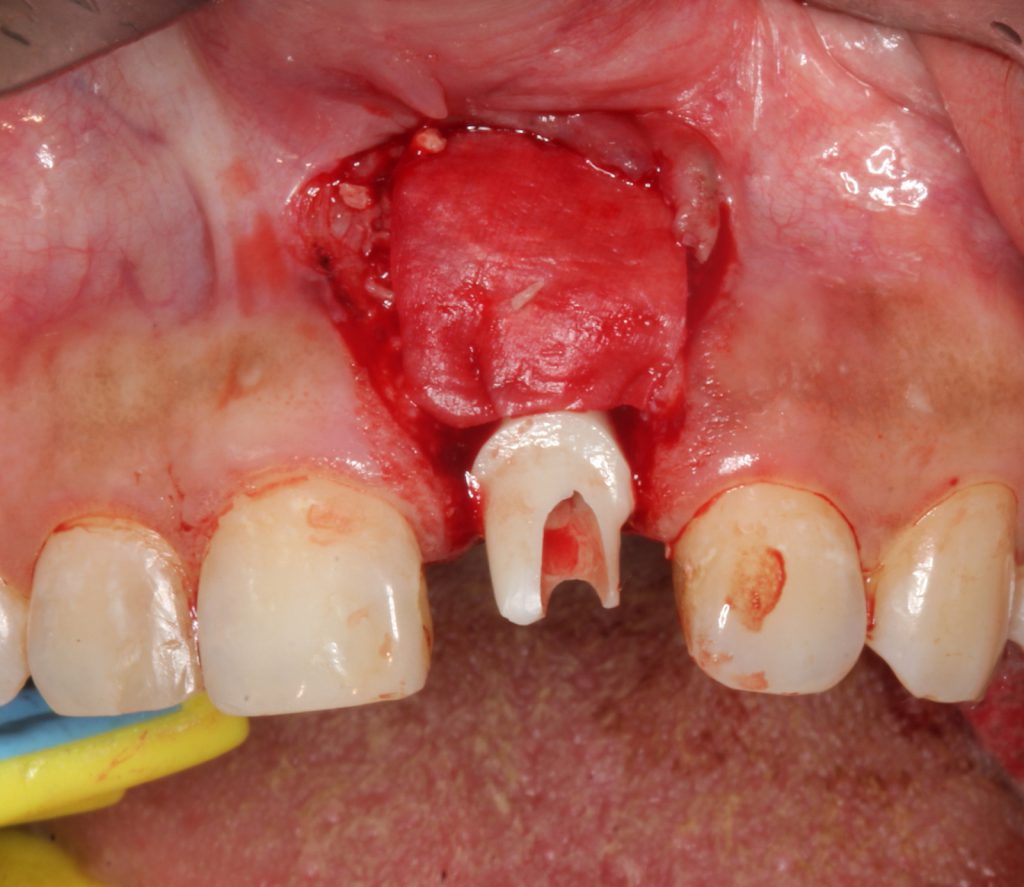
Figure 7. The area was deficient in both the vertical and horizontal bulk of keratinized tissue, and a connective tissue graft harvested from the palate was placed over the bony matrix.
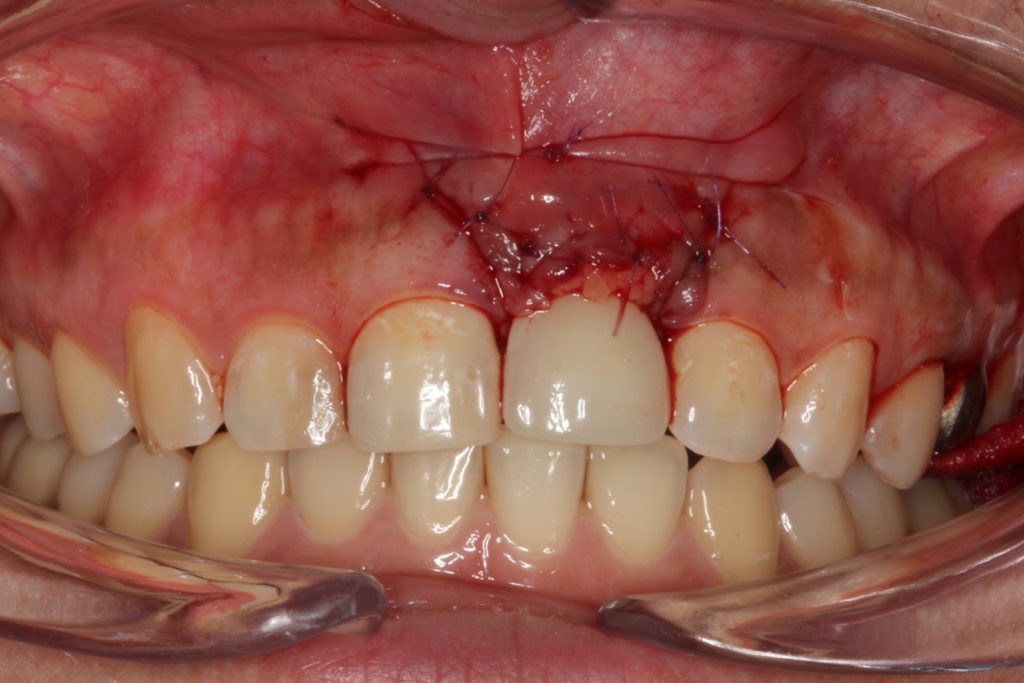
Figure 8. Closure was accomplished with sutures and the established placement of the flap coronally to the wound area.
For postoperative care, an oral microbial rinse (PerioSciences) was utilized by the patient along with a systemic antibiotic of amoxicillin 500 mg for 10 days. The patient was instructed not to brush the affected area for 2 weeks. Sutures were removed at the 6-week post-op appointment. The patient was seen on a periodic basis for over one year.
At the one-year followup, the abutment was covered with a healthy gingival biotype of thick keratinization (Figure 9). One-year radiographs demonstrated possible osseous regeneration around the implant platform with an excellent long-term prognosis (Figure 10).
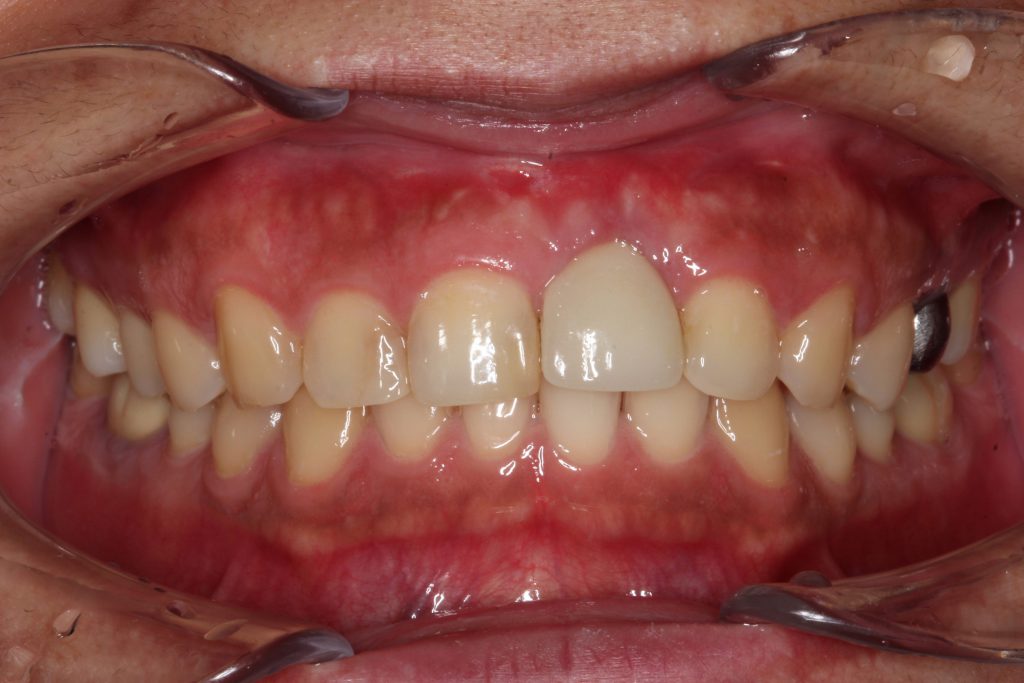
Figure 9. At one year, the healing was excellent with minimal pocket depth, no suppuration, and an adequate zone of keratinized gingiva.
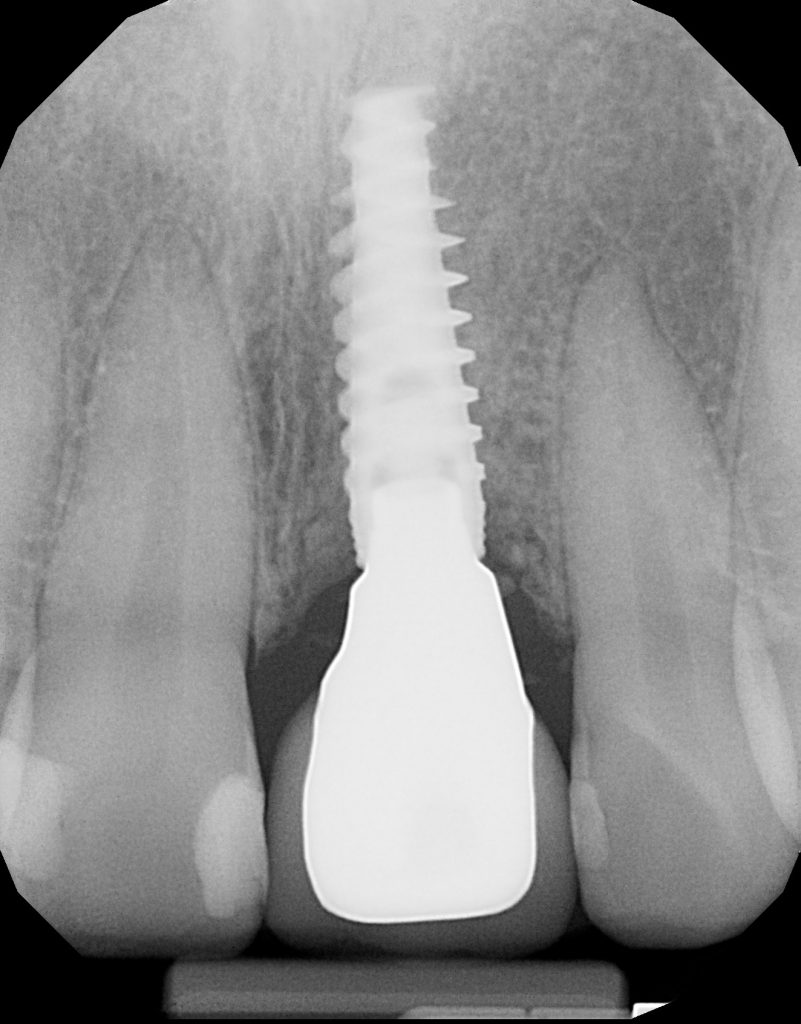
Figure 10. A radiograph at one year demonstrated minimal resorption around the implant threads and stabilization of implant health.
Case 2: Managing Moderate to Severe Peri-implantitis
A 66-year-old male patient had a chief concern of “gum swelling around my implant.” The implant for the maxillary left canine had been placed 12 years previously. There was a diagnosis of early peri-implantitis (2 years after the implant was restored) with bleeding around the implant and significant pocket depth. The patient elected not to have treatment at that time. Presently, upon observation, there was a severe gingival abscess on the facial aspect with suppuration upon palpitation (Figure 11).
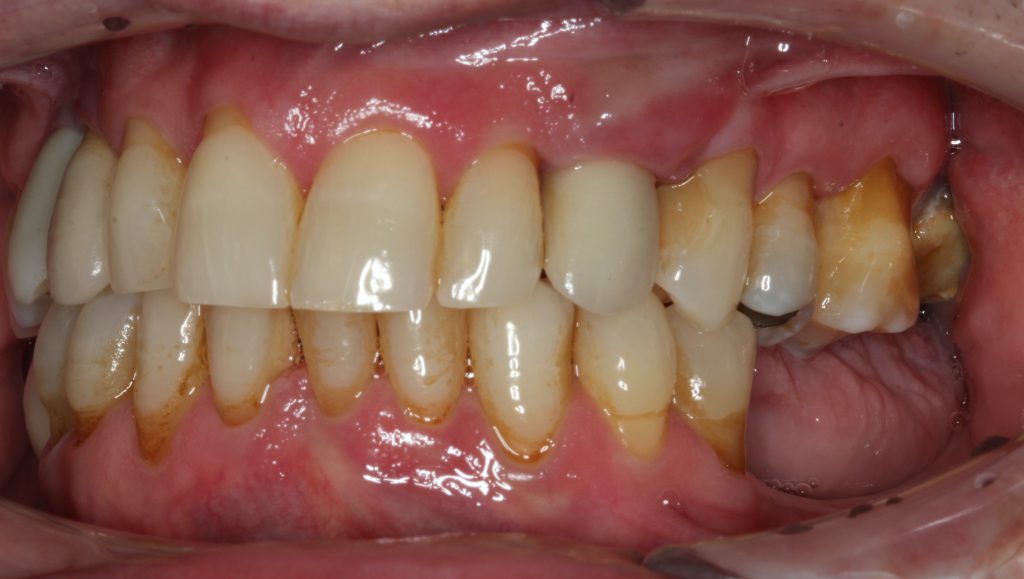
Figure 11. The patient presented with a gingival abscess around a maxillary left canine with bleeding on gentle probing and substantial suppuration on palpation. The pocket depths were in excess of 10 mm.
The radiograph demonstrated severe osseous resorption and pocket depths of more than 10 mm at the mesial and distal sites of the canine implant with involvement of the adjacent natural dentition (Figure 12). Clinically, Class III mobility was found on the maxillary left lateral tooth.
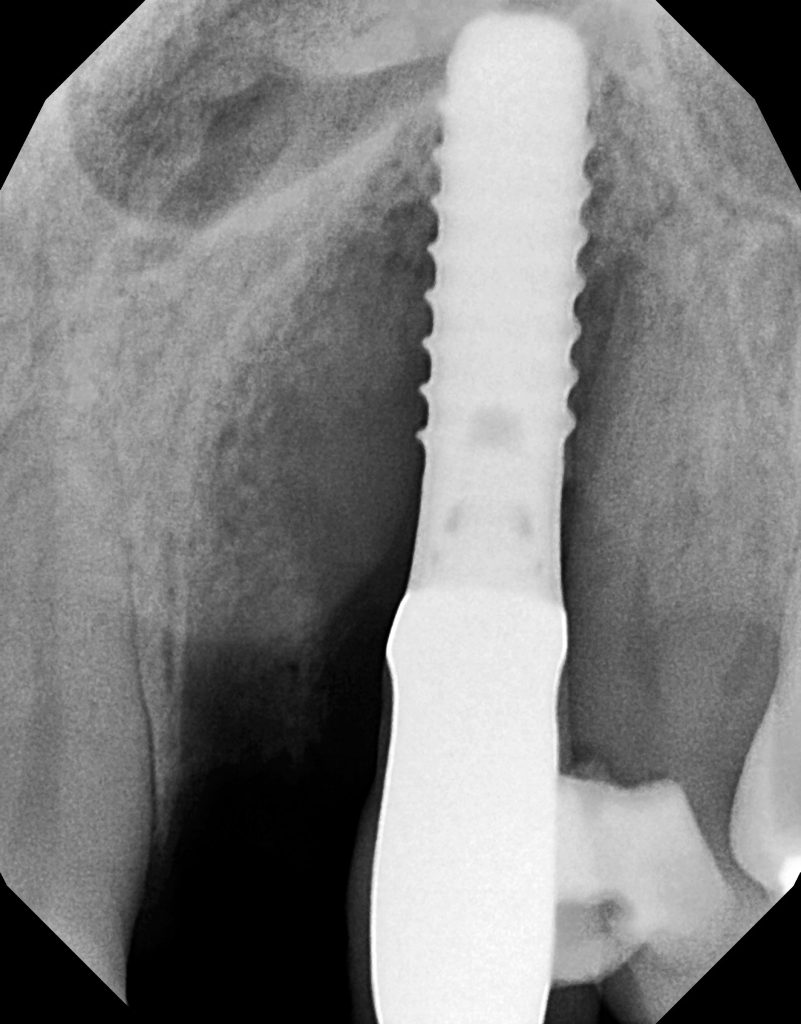
Figure 12. The radiograph demonstrated major osseous resorption circumferentially around the implant with a large 3-wall defect on the mesial site.
No mobility was observed on the maxillary left canine implant nor the maxillary left first premolar tooth.
The patient was adamant about not extracting the implant and desiring as minimal a loss of teeth as possible. Therefore, the clinician/author elected to extract the left maxillary lateral and repair the implant and the maxillary first premolar with biologic regeneration and a laser. If the procedure achieved the desired results, then a cantilever prosthesis would be considered.
The surgical repair implant procedure included external de-epithelization with the Er,Cr:YSGG laser to exclude epithelial migration into the subsequent wound. Prior to the incision, the sulcular epithelium was removed with the laser. After an inverse bevel sulcular incision and a vertical incision at the distal of the premolar, a full-thickness flap was reflected.
The lateral tooth was removed without complications (Figure 13).
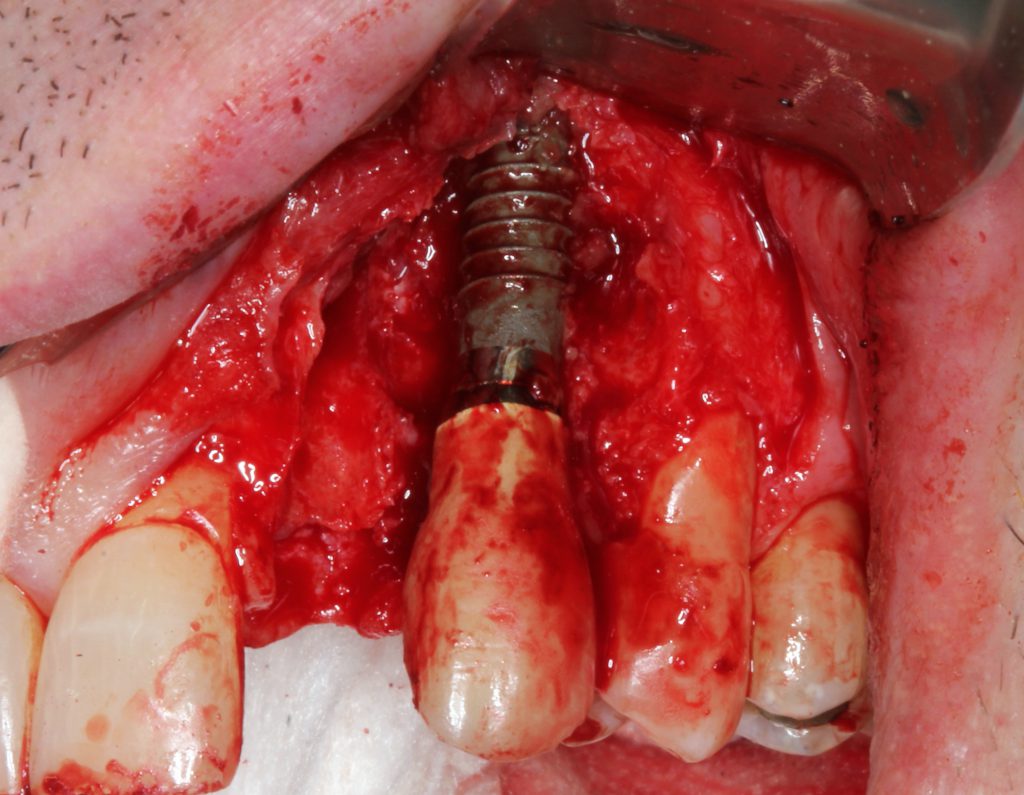
Figure 13. Vertical incisions were performed to provide access to the implant site. Significant granulation tissues were associated with the bony defect.
All granulation tissue was removed with the radial-firing laser tip revealing significant osseous resorption around the implant surfaces (Figure 14). The implant surfaces were decontaminated with the sapphire side-firing laser tip. The maxillary first premolar root was also managed with a repair laser procedure including ultrasonic and manual instrumentation, followed by smear layer removal with the laser using a radial-firing tip.
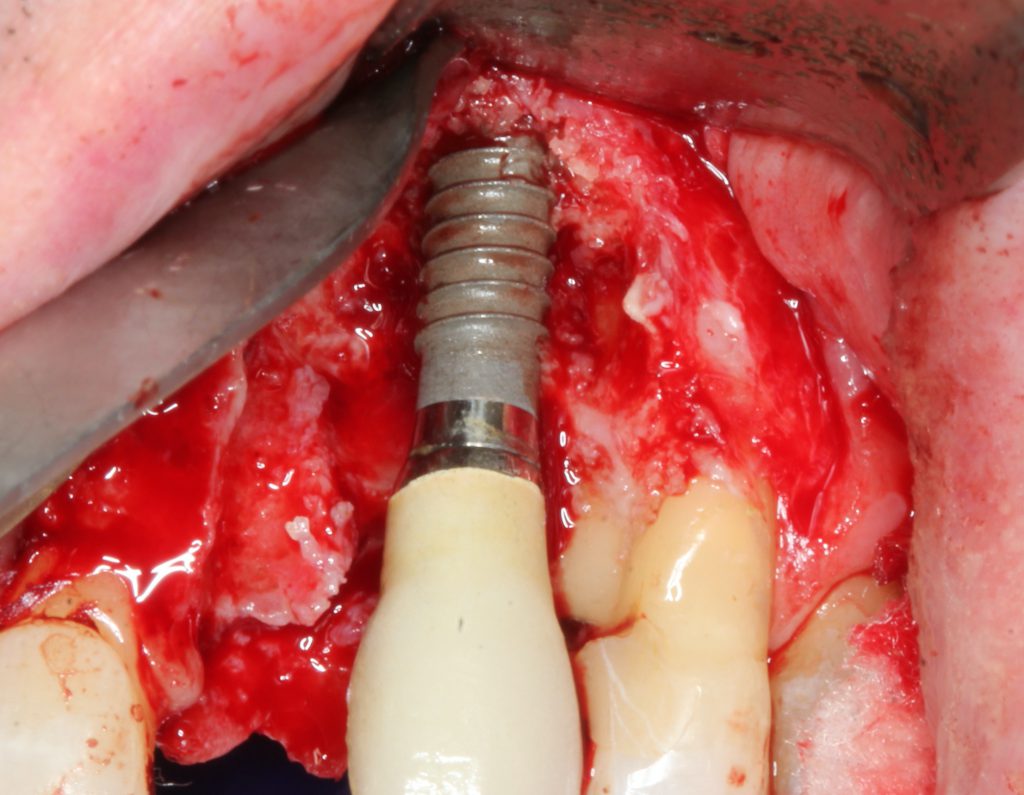
Figure 14. The Er,Cr:YSGG laser with a radial-firing tip was utilized to remove the granulation tissue. The side-firing tip directed energy at right angles into the pitch of the threads for decontamination.
The entire defect area was de-corticated using an end-firing tip to promote osteogenesis.
For augmentation, mineralized freeze-dried bone allograft was mixed with platelet-rich fibrin (PRF) and secured with an amnion/chorion membrane with a PRF membrane on top (Figure 15). The flap was then replaced with a non-resorbable, monofilament suture (Figures 16 and 17). PBM was achieved with the 940-μm diode laser.
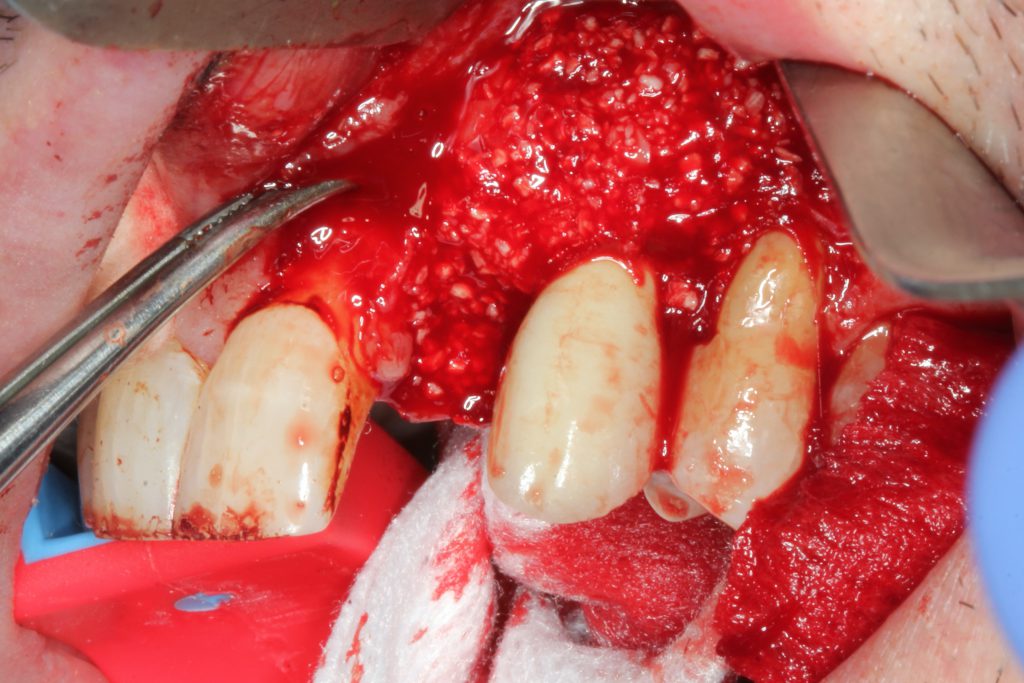
Figure 15. Due to the angular osseous architecture, a mineralized, freeze-dried bone allograft was mixed with platelet-rich fibrin (PRF) and secured with a PRF membrane, then placed in the defect after decortication with an end-firing tip and Er,Cr:YSGG laser.
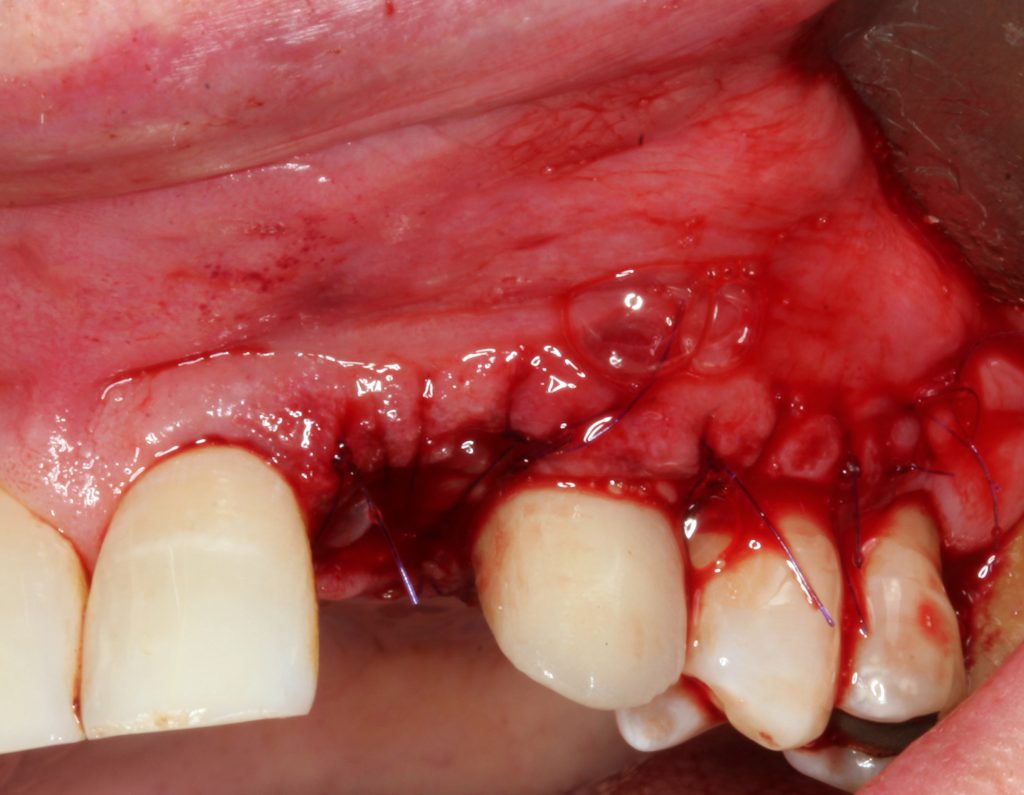
Figure 16. The area was closed with a non-resorbable monofilament suture, and photo biomodulation was achieved with a 940-μm diode laser (Epic X [BIOLASE]).
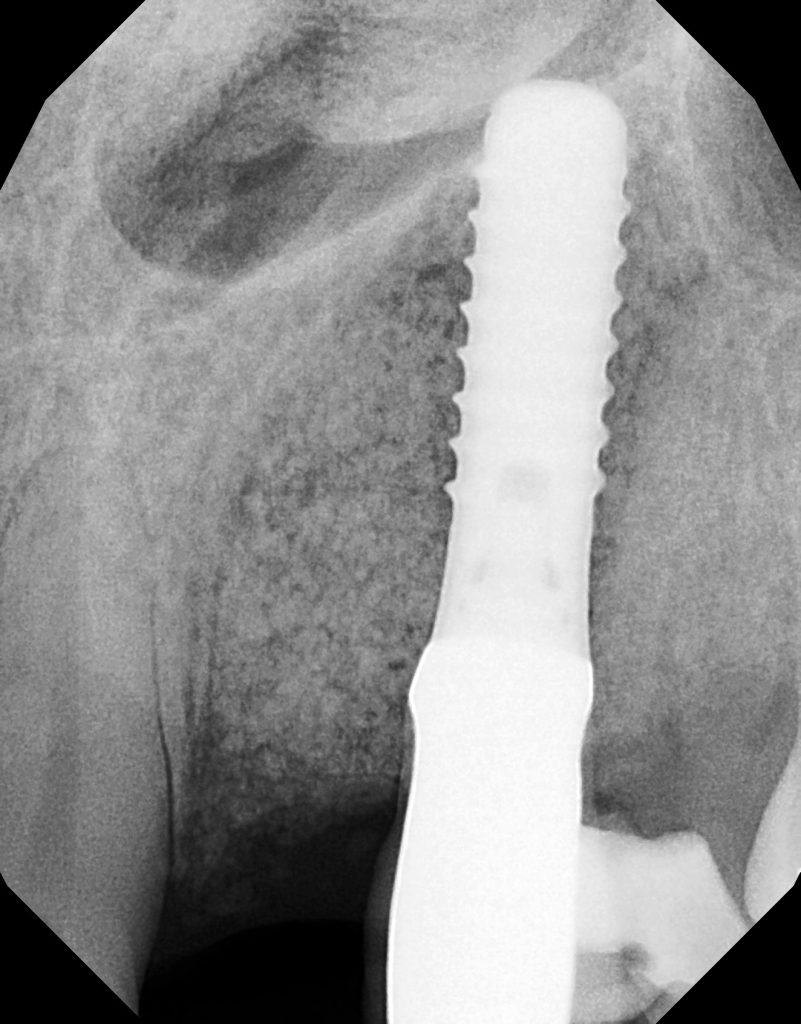
Figure 17. An immediate postoperative radiograph was taken demonstrating correct positioning of the bone graft.
An antimicrobial rinse and amoxicillin were administered post-op. The patient returned for periodic debridement utilizing the guided biofilm therapy protocol with an erythritol air delivery device (AIRFLOW Prophylaxis Master [EMS]). At 6 months, the radiograph and clinical evaluation appeared to demonstrate osseous regeneration, and the patient was scheduled for a provisional implant cantilever prosthesis (Figures 18 and 19).
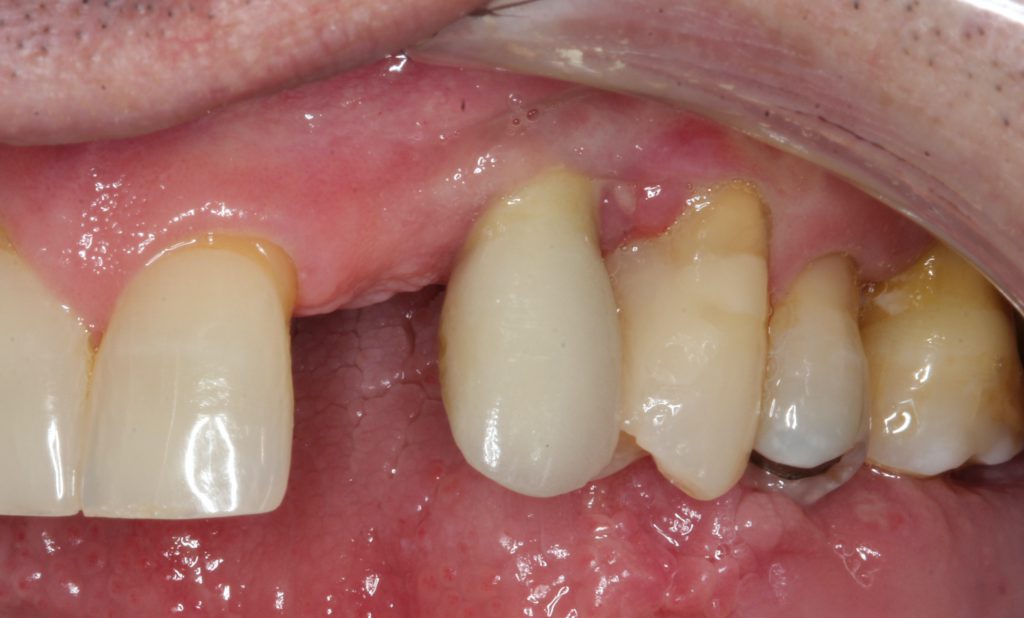
Figure 18. At the 6-month post-op appointment, the wound healing was acceptable, with no suppuration and minimal inflammation.
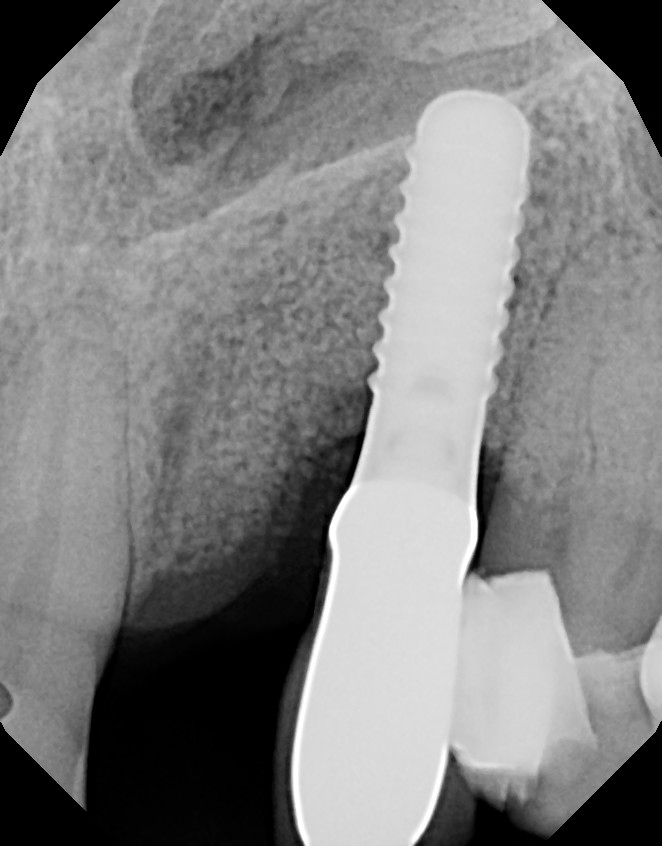
Figure 19. The 6-month radiograph shows stabilization of the osseous augmentation.
At one year, a final restoration will be completed with the 1-year post, demonstrating osseous regeneration around the implant (Figures 20 and 21).
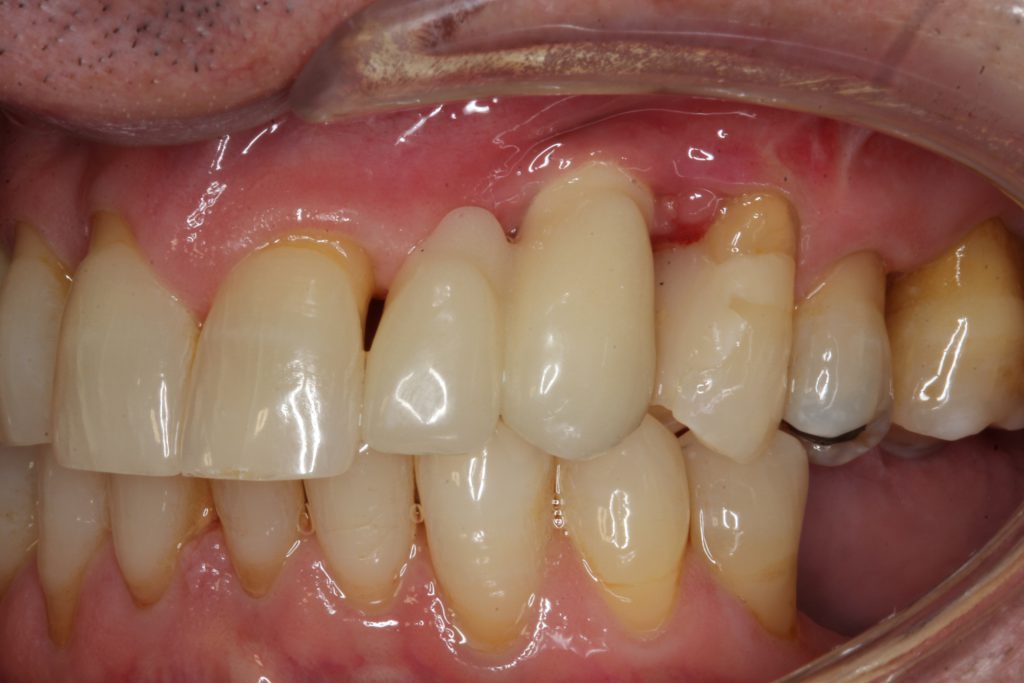
Figure 20. A provisional restoration was fabricated with a cantilever from the maxillary left canine replacing the maxillary left lateral.
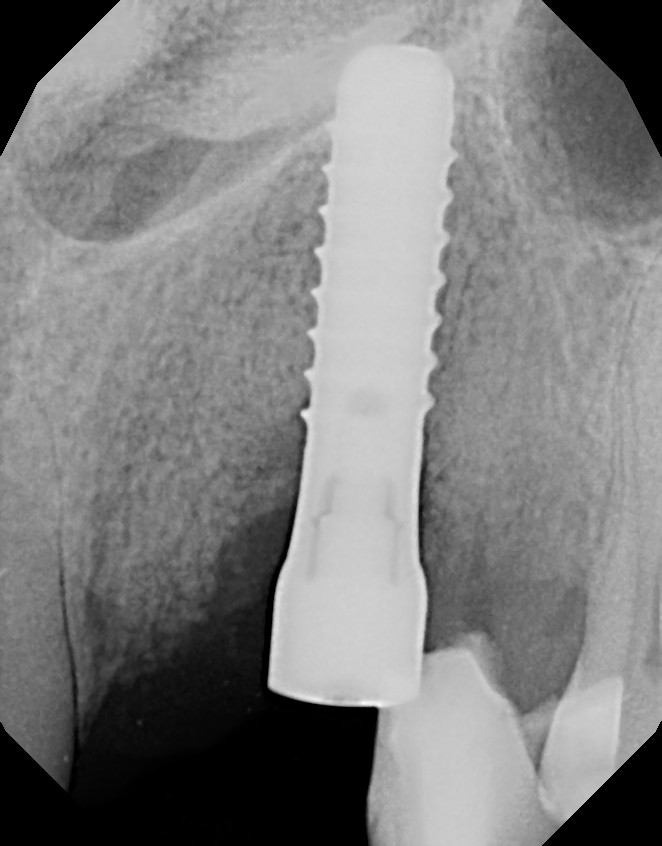
Figure 21. The final radiograph demonstrated continued osseous fill around the maxillary left canine implant.
CONCLUSION
These case studies demonstrate that the Er,Cr:YSGG laser can create a receptive environment for both biologic and osseous grafting material to enhance the regeneration of osseous structure and allow for a quality bone implant contact. The Er,Cr:YSGG laser can effectively degranulate inflammatory tissue, decorticate osseous structures, and decontaminate titanium implant surfaces with no adverse events and, thus, enhance a wound-healing regenerative environment.
While in the past with peri-implant disease, the clinician had minimal options to manage diseased implants, we now can enhance the prognosis by utilizing laser technology in combination with regenerative materials to save dental implants.
REFERENCES
1. Daubert DM, Weinstein BF, Bordin S, et al. Prevalence and predictive factors for peri-implant disease and implant failure: a cross-sectional analysis. J Periodontol. 2015;86(3):337–47. doi:10.1902/jop.2014.140438
2. Schwarz F, Derks J, Monje A, et al. Peri-implantitis. J Clin Periodontol. 2018;45 Suppl 20:S246-S266. doi:10.1111/jcpe.12954
3. Yamamoto A, Tanabe T. Treatment of peri-implantitis around TiUnite-surface implants using Er:YAG laser microexplosions. Int J Periodontics Restorative Dent. 2013;33(1):21-30. doi:10.11607/prd.1593
4. Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol. 2015;86(1):9-15. doi:10.1902/jop.2014.140363
5. Danesh-Sani SA, Tarnow D, Yip JK, et al. The influence of cortical bone perforation on guided bone regeneration in humans. Int J Oral Maxillofac Surg. 2017;46(2):261–6. doi:10.1016/j.ijom.2016.10.017
6. Clem D, Gunsolley JC. Peri-implantitis treatment using Er:YAG laser and bone grafting. A prospective consecutive case series evaluation: 1 year posttherapy. Int J Periodontics Restorative Dent. 2019;39(4):479–89. doi:10.11607/prd.4158
7. Nevins M, Nevins ML, Yamamoto A, et al. Use of Er:YAG laser to decontaminate infected dental implant surface in preparation for reestablishment of bone-to-implant contact. Int J Periodontics Restorative Dent. 2014;34(4):461–6. doi:10.11607/prd.2192
8. Nevins M, Benfenati SP, Galletti P, et al. Human histologic evaluations of the use of Er,Cr:YSGG laser to decontaminate an infected dental implant surface in preparation for implant reosseointegration. Int J Periodontics Restorative Dent. 2020;40(6):805–12. doi:10.11607/prd.5139
ABOUT THE AUTHORS
Dr. Low received his DDS and MS degrees from the University of Texas at Houston. He also completed his residency in periodontics at the University of Texas at Houston and received an MEd degree from the University of Florida. He was named vice president, dental and clinical affairs, and chief dental officer of BIOLASE in October 2016.
He is professor emeritus at the University of Florida College of Dentistry and associate faculty member of the Pankey Institute and has 30 years of private practice experience in periodontics, lasers, and implant placement. He is a Diplomate of the American Board of Periodontology and past president of the American Academy of Periodontology. Dr. Low is also a past president of the Florida Dental Association and a past ADA trustee. He can be reached at slow@dental.ufl.edu.
Disclosure: Dr. Low is chief dental officer, BIOLASE, Inc, and a consultant for PerioScience and EMS.
Dr. Chang is a graduate of the University of Maryland Dental School and practices in McKinney, Texas. He obtained his certificate in periodontics and prosthodontics through a 5-year residency program at the University of Texas Health Science Center, San Antonio. Dr. Chang is a diplomate of the American Board of Prosthodontics and the American Board of Periodontology. He can be reached at pchangdds@gmail.com.
Disclosure: Dr. Chang receives honoraria from BIOLASE, Inc, and is a speaker and trainer for the company.