The use of facemasks was first advocated at the end of the 19th century. They were initially designed to reduce the chances of postsurgical infections in patients. The goal was to prevent or reduce the numbers of microorganisms released from the respiratory tracts of the surgical team. However, controversy exists concerning the ability of masks to decrease the number of potential pathogenic microorganisms. It has never been shown that wearing a facemask by healthy individuals resulted in fewer nosocomial infections. Benefit has been noted, however, when the wearer had a sore throat.1-3
There are several reasons how a surgical mask could contribute to the contamination of surgical wounds. If the tension on the attachment strings is inadequate, venting could occur. Venting is leakage of air from the edges of the mask. Moist, exhaled air increases airflow resistance, which is thought to increase the chances of venting. As masks become moist, the chance of wicking increases. Wicking is a process of conveying liquid via capillary action. This could aid the passage of microorganisms. When worn, masks rub against the users skin, causing friction. This is called wiggling and has been shown to cause dispersal of skin scales. If a mask is worn incorrectly, the nose and mouth could be exposed. Grasping the filter section of a mask could result in contamination of hands or gloves or decrease the efficiency of the mask itself.4-6
Wearing masks that cover both the mouth and the nose during surgical procedures has been a routine procedure for almost 90 years. Today, masks are also commonly worn during nonsurgical medical procedures. This is especially valid when airborne-spread diseases are present in a population.2,5
PROTECTING THE PRACTITIONER
In recent years, facemasks have also been viewed as an important means to protect healthcare workers (HCWs) from potential respiratory disease agents. The primary source of such pathogens is the patient. Sprays, splashes, and some aerosols of body fluids and other potentially infectious materials can be involved. Speaking, coughing, and sneezing also release organisms into the immediate environment.3,6-9
Table 1. Compliance with the revised OSHA bloodborne pathogens standard*
|
The Occupational Safety and Health Administration (OSHA), in its Bloodborne Pathogens Standard, recognizes the importance of protecting HCWs from occupational exposure to the blood and other potentially infectious materials of patients. Protection of HCWs can be afforded in 3 basic methods or processes (see Table 1).8
The most effective method of protection employs engineering and work practice controls. Example activities include processes that assure routine emission of acceptable dental-unit water, the use of rubber dams and high-volume evacuation, and the application of pre-procedural mouth rinses.8,9
Where occupational risk remains after institution of these engineering and work practice controls, personal protective equipment (PPE) should be used. The employer is required to provide PPE at no cost to the employee. Masks are considered “appropriate” PPE only if they do not permit blood and other potentially infectious materials to pass through to or reach skin or the mucous membranes of the nose and mouth. Protection can only be expected under normal conditions of use and for the duration of time for which a mask was designed. Differing performance expectations require different types of masks.8
Under OSHA standards, the employer must ensure that employees use appropriate PPE correctly. The employer is also required that PPE (in this case, masks) be readily accessible in the correct sizes and proper types for the hazards present.8
ABOUT MASKS
Table 2. Characteristics of surgical masks*
|
The FDA is responsible for regulating medical devices. Surgical masks are examples of a regulated FDA medical device. Characteristics used to describe surgical masks are presented in Table 2. Surgical masks are primarily tested in laboratory situations, not in actual use on HCWs.
Surgical masks are disposable and are composed of multiple layers of synthetic (microfiber) filter materials designed to collect and retain microscopic particles. The minimum goal is to filter out at least 95% of small particles that directly contact the mask.3,8,9,14
In 1995, the National Institute for Occupational Safety and Health (NIOSH) began to certify 3 classes of filters—the N, R, and P series, each with 3 levels of filter efficiency (95%, 99%, and 99.9% respectively). NIOSH is the federal agency responsible
for conducting research and making recommendations for the prevention of work-related injury and illness. It is part of the CDC in the Department of Human Health and Safety.3,8,9,14
Masks come in a variety of shapes and sizes. Some masks are preformed domes, while others are more pliable. Masks are secured to the user by elastic bands, ear loops, or some type of tie. Most masks are form-fitted over the bridge of the nose and cheeks to reduce fogging by warm, expelled air.3,6,13
USING MASKS
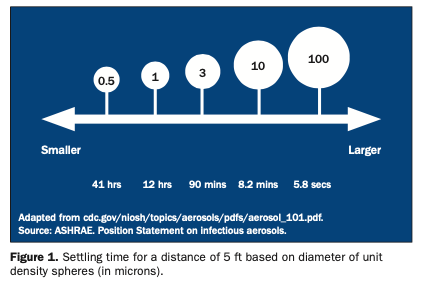
Bacterial filtration efficiency (BFE) and particulate filtration efficiency (PFE) values are important. BFE measures the filtering of bacteria that range in size from 1 to 5 microns (um). Microns are one thousandth of a millimeter. PFE measures much smaller (0.1 to 1 um) particulate (nonviable) matter. When considering a surgical mask, BFE and PFE values (percent retained) and the size of the particle upon which the values are based are important. In order to have the proper mask for a given application, several different types and sizes of masks must be made available.3,10-13
Even with high filtering efficiency, some exhaled air can escape unfiltered around the edges of the mask. The greater the edge leakage of a mask, the lower will be the actual in-use BFE and PFE values. The bottom line is that a mask is only as good as it fits. In order to accommodate the fit of several sizes and shapes of faces, more than one type and size of surgical facemask should be present in a practice.5
Breathability, or Delta P, measures the pressure drop across a facemask. The higher the Delta P values, the more difficult the mask is to breathe through. Persons with breathing difficulties should use masks with lower Delta P values.10-13,9
Fluid resistance measures a facemasks ability to minimize fluids traveling through the material. The greater the fluid resistance of a mask, the lower will be the potential exposure to blood and body fluids caused by splashes, spray, and spatter. Surgical masks are available with fluid-resistant outer layers and tissue inner layers or fluid-resistant outer and inner layers. Selection as to fluid resistance is influenced by the procedures being conducted and personal preference.10-13
For the vast majority of dental procedures, a surgical-type mask with a >95% bacterial filtration efficiency should be used. Such masks are sufficiently protective against aerosols and large droplet spatter.3,8,9,13
Masks should be changed after every 20 minutes of heavy exposure to fluids or after an hour of normal use. Masks become less effective the wetter they become. Surgical masks are considered to be single-use, disposable items and should be discarded after each patient treatment. They should be removed by touching only the ties, bands, or loops.6,11
REGULATIONS AND RECOMMENDATIONS
OSHA requires that masks combined with eye protection devices such as goggles or spectacles with solid shields or full-length face shields be worn whenever splashes, spray, spatter, or droplets of blood or other potentially infectious materials may be generated, and when eye, nose, or mouth contamination can be reasonably anticipated.8
The new CDC infection control guidelines also address the issue of masks. The CDC recommend that a surgical mask and eye protection with solid side shields or a face shield be worn to protect mucous membranes of the eyes, nose, and mouth during dental procedures likely to generate splashing or spattering of blood or other body fluids.9
Adverse Reactions
Wearing surgical masks is not without risk. Masks can irritate facial skin by friction. Facemask material coloring (dyes) and printing can also cause irritation or even hypersensitivity. Persons with sensitive skin may be better served through the use of masks with white outer layers and white, nonprinted inner layers.6,11,12
Materials used to fabricate surgical masks can also cause hypersensitivities. Latex substances, including adhesives containing latex, may be present. The metal strip or bar used to fit a mask better to a user’s face can be problematic. In a limited number of cases, metals can be released and cause difficulties.2,3,9-12
Tuberculosis
When airborne infection control precautions are necessary (eg, for tuberculosis patients) a NIOSH-certified particulate-filter respirator (eg, N95, N99, or N100) should be used. N95 refers to the ability of a filter to retain 1-um particles in an unloaded state with a filter efficiency of >95% (with a 5% leakage). The flow rate assumed is ≤50 liters/
minute, which is thought to be the maximum airflow rate produced by a HCW during breathing. Current research indicates that infectious droplets nuclei involved measure between 1 and 5 um. N95 respirators, when properly tested and fitted correctly, should be adequate for the situation.9,15
The majority of surgical facemasks used in dentistry are not NIOSH-certified respirators. Wearing such masks is not protective and does not meet OSHA requirements for respiratory protection. However, there are some surgical masks (surgical N95 respirators) that do meet the requirement and are certified as being respirators by NIOSH.9,12,15
Fortunately, N95 respirators are not often required. Detailed information regarding airborne transmission precautions and respirator programs, including fit-test procedures, are available at cdc.gov/niosh/99-143.html.
References
1. Tunevall TG. Postoperative wound infections and surgical face masks: a controlled study. World J Surg. 1991;15:383-388.
2. Rockwood CA Jr, O’Donoghue DH. The surgical mask: its development, usage, and efficiency. A review of the literature, and new experimental studies. Arch Surg. 1960;80:963-971.
3. Miller CH, Palenik CJ. Infection Control and Management of Hazardous Materials for the Dental Team. 3rd ed. St Louis, Mo: Mosby; 2004: 283-288.
4. Tunevall TG, Jorbeck H. Influence of wearing masks on the density of airborne bacteria in the vicinity of the surgical wound. Eur J Surg. 1992;158:263-266.
5. Belkin NL. The evolution of the surgical mask: filtering efficiency versus effectiveness. Infect Control Hosp Epidemiol. 1997;18:49-57.
6. Lipp A. A guide to developing a systematic review. AORN J. 2003;78:90-107.
7. Prospero E, Savini S, Annino I. Microbial aerosol contamination of dental healthcare workers faces and other surfaces in dental practice. Infect Control Hosp Epidemiol. 2003;24:139-141.
8. US Department of Labor, Occupational Safety & Health Administration. 29 CFR Part 1910.1030 Occupational exposure to bloodborne pathogens; Needlestick and other sharps injuries; Final rule. Federal Register. 1991;56:64175-64182 and 2001;66:5317-5325. Also available at: http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=STANDARDS&p_id=10051. Accessed December 2003.
9. Kohn WG, Collins AS, Cleveland JL, et al; Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
10. Miller CH, Palenik CJ. Sterilization, disinfection and asepsis in dentistry. In: Block SS. Disinfection, Sterilization, and Preservation. 5th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2000: 1049-1068.
11. Rawson D. The basics of surgical mask selection. Available at: http://www.infectioncontroltoday.com/articles/331feat2.html. Accessed December 2003.
12. Andrews N, Molinari JA. Meeting the challenge of airborne diseases. J Prac Hygiene. 2003;129(4):25-26.
13. Whitehead AG. The most important barrier. Dent Equip & Mater. 2003;8(8):76,78.
14. Whitehead AG. Your most important barrier. Dent Econ. 2002;92:138,154.
15. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. MMWR Recomm Rep. 1994;43(RR-13):1-132.
Dr. Palenik has held over the last 25 years a number of academic and administrative positions at Indiana University School of Dentistry. These include professor of oral microbiology, director of human health and safety, director of central sterilization services, and chairman of infection control and hazardous materials management committees. Currently, he is director of infection control research and services. He has published 125 articles, more than 290 monographs, 3 books, and 7 book chapters, the majority of which involve infection control and human safety and health. Also, he has provided more than 100 continuing education courses throughout the United States and in 8 foreign countries. All questions may be directed to OSAP by e-mail at office@osap.org.
Ms. Govoni is a certified and registered dental assistant and a registered dental hygienist with more than 31 years of experience in the dental profession as a chairside assistant, office administrator, clinical hygienist, educator, consultant, and speaker. She is the owner of Clinical Dynamics, a consulting company dedicated to the enhancement of the clinical and communication skills of dental teams. She is a past president of the American Dental Assistants Association, a member of the Organization for Safety and Asepsis Procedures, the American Dental Hygienists Association, and the National Speakers Association, and has served on the Michigan Board of Dentistry. She is also a columnist for Dental Equipment and Materials and is a featured speaker on the ADA Seminar Series.