Dental healthcare professionals historically have been able to meet numerous infection control challenges, ranging from the threats of bloodborne infections like hepatitis B and AIDS to the re-emergence of tuberculosis. Numerous effective options are available in the marketplace to address and accomplish infection control goals. It is not unusual for dentists, hygienists, assistants, and laboratory technicians to occasionally feel overwhelmed and possibly a bit confused when reading published guidelines and regulations, especially when we all want to be assured that what is being done is effective and in compliance with professional standards. Fundamental to this process is having a working comprehension of infection control principles.
In this article, Dentistry Today’s editor-in-chief, Dr. Damon Adams, poses many of the questions asked by dental healthcare professionals.
 |
| John A. Molinari, PhD |
Dr. Molinari, it seems that many people are confused about requirements for sterilization monitoring. What are the requirements?
The US Centers for Disease Control and Prevention (CDC) recommends that correct functioning of sterilization cycles should be verified for each sterilizer using a biological indicator (BI) and control at least once a week.1 Virtually every state has taken this quality control recommendation from the CDC and turned it into a requirement for most healthcare settings, including dental practices. In addition, loads containing implantable devices should be biologically monitored, and the items quarantined until BI results are known. The importance of routine biological monitoring cannot be overemphasized, as BIs can ascertain the effectiveness of many of the steps involved in instrument reprocessing. Sterilization procedures are routinely monitored using a combination of mechanical, chemical, and biological indicators. Taken together, these indicators evaluate the sterilizing conditions and effectiveness of the process. Because BIs directly monitor sterilization by assessing the processes’ ability to kill known highly resistant bacterial spores (eg, Geobacillus or Bacillus species), rather than only testing the physical and chemical conditions necessary for sterilization, the use of calibrated BIs remains the accepted “gold standard” for monitoring.
Although multiple studies have shown that the most common cause of sterilization failures is human error, other problems can compromise sterilizer efficiency. A few common errors are listed in Table 1.
We have seen advertisements that tout the use of “faster” sterilizers; how are they different from the standard autoclave and what are the advantages of “faster and better”?
If you are using an autoclave like the overwhelming majority of dental practices, you probably have been using a gravity displacement autoclave. This type of unit has been available for more than a century and sterilizes with self-generated steam that is created within the chamber or by a component steam generator. Because air entering the chamber mixes with air, cool air pockets can form within the chamber, which may result in extended times for sterilization of certain items. In addition, overloading this type of autoclave can lead to incomplete drying of sterilized instrument packs, resulting in the user commonly finding wet packages at the end of the cycle. Modification of later generations of autoclaves has resulted in some sterilizers using pressure-pulsing techniques along with gravity displacement techniques to assist in removing air from the chamber before the sterilization cycle.
Development of a new generation of autoclaves within the last 2 decades has added a new dimension to this heat sterilization modality. These autoclaves are classified as “Class B” sterilizers or “pre- and post-vacuum” steam sterilizers. The equipment is fitted with a pump that creates an initial vacuum in the chamber to ensure air is removed from the sterilizing chamber before steam enters. In contrast to a gravity displacement autoclave, this procedure allows faster and more thorough steam penetration throughout the entire load. The poststerilization vacuum cycle also is highly efficient because it facilitates drying. Practices that utilize this type of sterilizer frequently report the instrument packs are “bone dry” at the end of the sterilization process.
| Table 1. Common Process Errors Seen In Sterilization Cycle Failure |
|
If you could present people with fundamental rules of thumb for practicing proper infection control in a dental office, what would they be?
One aspect of infection control that is sometimes forgotten in the ongoing evolution of technologies, methodologies, and products is that long-standing, basic infection control principles have not really changed over the years. While there is certainly no single “best list,” I can offer the following to summarize major areas:
- Perform effective hand hygiene
- Immunize against vaccine-preventable diseases
- Use personal protective equipment appropriately
- Heat sterilize all reusable patient care instruments/items used intraorally
- Use respiratory hygiene/cough etiquette
- Prevent cross-contamination with aseptic technique and environmental asepsis
- Prevent sharp injuries by using safe work practices and engineering controls.
You may find that as you review and consider each of these, many of the procedures and protocols that you perform as routine components of your practice day provide effective applications of these principles. You may be pleasantly surprised as to just how well your infection control program is working.
 |
| Demonstration of fit and feel for right- and left-fitted versus ambidextrous gloves. |
There are new products on the market that talk about being “eco-friendly,” yet many infection control products are labeled as disposable, and I hear some may even be toxic to the environment. What is the status of environmentally friendly infection control products?
A new era of developing strategies and marketing products designed to address environmental issues is rapidly expanding into multiple areas of infection control. Many hospitals and other healthcare facilities took the initiative to lower waste accumulation by using approaches that include adopting programs that use more recyclables and reusable items and looking for products that reduce the amount of disposable waste.
“Green dentistry” has also become more than merely a slogan in the profession. A number of manufacturers are focusing on the use of disposable items. Widespread use of disposable covers, tips, traps, instrument wraps, pouches, and other items has helped to streamline infection control with regard to time and reprocessing efforts. Advances in research into the cost effective manufacture of recyclable and biodegradable plastic and paper items appear to be accelerating, with the hope that what was once destined for deposit in landfills may now be recycled and used again, in a similar way that aluminum cans are reprocessed. You may also be surprised to find that more “plastic” wraps and covers that we frequently use are manufactured to be biodegradable. This technology has also made its appearance into the areas of instrument reprocessing and environmental surface disinfection. Cleaning solutions used on contaminated instruments before heat sterilization can now contain multiple enzymes to enhance removal of bioburden. These components are biodegradable, thereby preventing introduction of potentially harmful chemicals into ground water. Even the relatively recent application of environmental surface disinfectant solutions and wipes is seeing continued progress towards reducing chemical waste. Tuberculocidal disinfectant preparations that are less harmful to the environment, even biodegradable, are already available, in addition to reusable plastic containers.
Dr. Molinari, with all of the recent stories in the media about the spread of infectious disease in healthcare settings, it is almost unbelievable that people are not following even the most basic guidelines. Why are these incidents occurring, and are they limited to healthcare facilities?
We continue to see reports about possible infectious disease transmission in hospitals and other healthcare facilities being traced back to glaring breaches in infection control. In just reviewing the past few years of possible incidents, multiple facilities have been investigated for failures associated with accepted infection control standards and instrument reprocessing protocols. Investigations also include 2 Veteran Affairs hospital dental clinics where the possible failure of clinic staff to comply with mandated infection control standards and protocols is under scrutiny. Allegations include improper use of gloves, burs, and procedures involved in instrument cleaning and sterilization of instruments.
From where do these and many other possible patient exposures result? Accumulated evidence gathered over many years has shown human error and failure to comply with required and/or recommended procedures to be the major culprits. Despite documentation of multiple instances, we still find some people, including healthcare professionals, who feel that taking shortcuts in certain infection control procedures is not particularly dangerous. However, please keep in mind that even the most rigorous infection control practices do not guarantee against accidental breaches or some undetected, undetermined, transmission incident. We simply must do the best we can to minimize the potential for cross-infection.
While the potential for infections may not be eliminated even when proper infection control practices are followed, lack of compliance can unknowingly increase the potential for microbial cross-contamination and cross-infection. What I think needs to be remembered is that proper application of individual infection control practices, procedures, and protocols help to strengthen other seemingly unrelated program components. Overlapping preventive measures are used routinely and they work. Sometimes we just do not think of them as being reinforcements for each other.
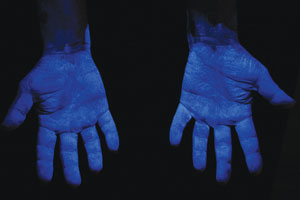 |
 |
| Hands coated with fluorescent powder representing contamination. | Residual powder remaining after brief wash procedure. |
As far as cleaning and disinfecting the operatory, what tips and suggestions do you have to make it efficient yet effective?
First and foremost, apply a basic premise of aseptic technique: clean it first. The importance of initial cleaning cannot be overemphasized and is included in all infection-control recommendations. Initial cleaning and subsequent disinfection are important, because together they minimize the potential for cross-infection from environmental surfaces. It also must be mentioned that manufacturers are required to state on the product label that the disinfectant should be applied onto precleaned surfaces. Although separate cleaners and disinfectants may be applied, chemical agents that accomplish both functions offer a more efficient approach. Recently published studies with THE DENTAL ADVISOR have reported the primary cleaning efficacy of commercial disinfectant sprays and wipes.
A few other suggestions on surface disinfection are offered in Table 2.2
Unfortunately, misuse or overuse of chemical disinfectants can create problems for both a person’s health and equipment integrity.3 In addition to discoloring or compromising the integrity of treated operatory surfaces, practice personnel can exhibit a variety of clinical manifestations from long-term unwanted exposure to chemicals. Respiratory problems, such as wheezing or sneezing, development of allergies, ocular irritation, and headaches can occur as a result of excessive spraying of a disinfectant. You may want to review and reevaluate written protocols for environmental asepsis if personnel in the practice develop these types of symptoms. The review should include consideration for implementing routine use of disinfectant wipes as a replacement for spray formulations.
| Table 2. Surface Disinfection Suggestions | |||||||||||||
|
What resources are available to dental healthcare professionals to stay up-to-date?
There are a number of Web sites and organizations available to provide you with additional information and current developments in this area. Two informative groups to consult initially can be found at osap.org and ecodentistry.org. Of course, dentaladvisor.com offers a “Q and A” area in the Infection Control Corner, where I respond to questions after my lectures and those posed by our readers.
Dr. Molinari, you recently accepted a new position as director of infection control with THE DENTAL ADVISOR in Ann Arbor, Michigan; can you comment on the work you are now doing?
I am honored to be part of such a great team of dental professionals. THE DENTAL ADVISOR has been providing scientific and evidenced-based research for almost 30 years. Our team meets weekly to discuss our publication, evaluations, long-term clinical studies, and to report on issues relevant to real world clinical dentistry. We work closely with manufacturers on product development and enhancement. Although I miss working with my students at the dental school, my role as director of infection control allows me to do what I love most: research, continuing to teach at different forums, and reporting to the dental profession. Our recently introduced sterilization monitoring program allows me to keep in contact with many offices regarding proper infection control practices, provide webinars, as well as provide subscribers access to our website 24/7, which has valuable information on all areas of clinical dentistry, as well as our research. Your readers may visit our infection control corner at dentaladvisor.com/clinical-evaluations/infection-control-corner.shtml to submit any questions related to infection control.
CLOSING COMMENTS
While dental care providers have much to be proud of in their infection control protocols and practices, new challenges continue to emerge that can potentially threaten our safety and that of patients. The past 30 years have seen a veritable explosion of science-, clinical-, and epidemiological-based knowledge in this area, with resultant development of new technologies and available product choices. As expected questions continue to be asked concerning the efficacy and potential problems of available infection control products, I deeply appreciate the opportunity to offer my thoughts in answering a few of these questions.
References
- Kohn WG, Collins AS, Cleveland JL, et al; Centers for Disease Control and Prevention (CDC). Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
- Molinari JA, Harte JA, eds. Cottone’s Practical Infection Control in Dentistry. 3rd ed. Baltimore, MD: Lippincott Williams & Wilkins; 2009.
- Rutala WA, Weber DJ; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for disinfection and sterilization in healthcare facilities. cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf. Accessed January 3, 2011.
Dr. Molinari is currently director of infection control for THE DENTAL ADVISOR in Ann Arbor, Mich. His position involves research on various products, publication of abstracts, and overseeing a Sterilization Monitoring Program, as well as lecturing and answering questions from readers of THE DENTAL ADVISOR. Previously, he served for 32 years at the University of Detroit Mercy School of Dentistry as professor and chairman of the department of biomedical sciences and director of infection control. He continues serving as a consultant for the CDC, ADA Council on Scientific Affairs, and the Council on Dental Practice. He is currently a member of the Michigan Board of Dentistry. In recognition of his efforts, Dr. Molinari was inducted as an honorary member of the Michigan Dental Association, the International College of Dentists, and the American College of Dentists, and he is a 2009 recipient of the ADA Golden Apple Award. He has published more than 350 scientific articles, text chapters, and abstracts, and he lectures internationally on infectious diseases and infection control. He has held many editorial positions for various publications and continues to serve the profession as an opinion leader on many topics related to infection control. He can be reached at (734) 665-2020 ext 110 or at jmolinari@dentaladvisor.com.
Disclosure: Dr. Molinari is a paid consultant for Hu Friedy Manufacturing as well as SciCan.