INTRODUCTION
An active clinician will encounter implant failure. Failures occur for myriad and interrelated reasons: limited inherent healing capacity, low bone quality, exacerbating patient factors (smoking, radiotherapy, diabetes, periodontitis, or other uncontrolled diseases), intraoperative or post-surgical infection, overloading, poor plaque control, a lack of keratinized mucosa, or bone deficits. The exact cause for non-integration or peri-implant tissue resorption in any given case may be ambiguous and multifactorial.1-4
We can curb these issues to a certain degree by avoiding implant treatment in medically compromised, smoking, orally unstable, or noncompliant patients and by ensuring ridge health prior to and during implant insertion or, at the latest, before restoration. The latter action may involve regenerating bone or keratinized mucosa in phases, placing implants as atraumatically as possible—eg, with bone expansion—and allowing for sufficient healing before moving to the next step. A fixture should be angled at a prosthetically ideal cant—preplanning with a diagnostic wax-up and using a surgical guide are key—but adequate bone must be created if that position is beyond the ridge envelope. If the bone cannot be built, then the implant should be placed at a non-ideal but prosthetically and hygienically acceptable angle or non-implant therapy should be recommended.5
Although time-consuming, staged surgery is advantageous, as it permits numerous opportunities for developing tissue. Such a protocol is especially advised for patients who wish to replace failing implants. Based on systematic reviews analyzing relatively few heterogenous, retrospective studies, the weighted mean survival rate of implants placed after one failure is 86% to 89% over 1 to 5 years; after 2 failures, the weighted mean survival of implants placed drops to 67% to 75%.6-8 As some authors have noted, the literature on this subject is highly variable and without controls, and there may be a moderate risk of bias.9,10
This paper presents 2 cases treating patients with first-time maxillary implant failures using a staged protocol. Performed recently, the first case incorporated more contemporary surgical methods, such as growth factors and motorized osteotomes.11 The second case took nearly 2 years to complete and involved a cross-arch reconstruction.
CASE REPORTS
Case 1: Right Premolars
A nonsmoking 63-year-old male with an American Society of Anesthesiologists (ASA) II physical status presented with complications at implants Nos. 4 and 5, which were placed by a general dentist. Concerned with integration, the original clinician referred the patient to a periodontist, who diagnosed No. 4 with severe peri-implantitis and removed the fixture.
A clinical and radiographic examination was performed at our practice (Figure 1). The ridge at Nos. 4 to 5 appeared to be significantly resorbed buccolingually. No keratinized mucosa was noted buccal to implant No. 5. A CBCT scan revealed a buccolingual dimension of 2 to 3 mm at the No. 4 ridge and 50% resorption of the labial plate at implant No. 5. At site No. 4, the distance from the maxillary sinus to the alveolar crest was 6.5 mm; at implant No. 5, this dimension was 13 mm. Implant No. 5 was tilted distally and angled at a 45° angle buccally.
The patient was diagnosed with severe buccolingual ridge resorption at No. 4; moderate maxillary sinus pneumatization at No. 4; peri-implantitis of No. 5; mucogingival defects at Nos. 5, 6, and 11; generalized mild periodontitis; occlusal trauma; and caries on teeth Nos. 2, 10, 12, and 14.
The initial treatment included full-mouth scaling and root planing, oral hygiene instructions, and referral to a restorative dentist for caries control and prosthetic analysis.
Regarding Nos. 4 and 5, an ideal diagnostic wax-up was created to guide prosthetically driven treatment. The plan included the removal of implant No. 5 with concomitant motorized osteotome-mediated internal sinus elevation and buccal guided bone regeneration (GBR), implant placement at Nos. 4 and 5 with further ridge augmentation as needed, second-stage implant exposure, and final restoration.
All surgery was performed under local anesthesia. One hour prior to each procedure, the patient took 500 mg of amoxicillin and 600 mg of ibuprofen. Postoperatively, the patient took ibuprofen 600 mg prn pain and amoxicillin 500 mg tid for 7 days. Full-thickness buccal and palatal flaps were raised, extended sulcularly around adjacent teeth as needed, and periosteally released; vertical releases were not performed.
Step 1: Removal of implant No. 5, sinus elevation and ridge expansion at No. 4, and buccal GBR at Nos. 4 and 5. The No. 5 implant was removed atraumatically by rotating it counterclockwise at 75 rpm with an attachment from an implant removal kit. At site No. 4, a pilot drill was used at 1,200 rpm with irrigation to a depth of 2 mm, and motorized osteotomes of sizes 1, 2, and 3 (BTI) were used at 75 rpm without irrigation to sequentially expand the ridge and elevate the maxillary sinus by 4 mm (Figures 2 and 3). Following sinus elevation, 0.5 g of cancellous particulate bone saturated in recombinant human platelet-derived growth factor-BB (rh-PDGF-BB) (Gem 21S [Lynch Biologics]) was inserted. GBR for buccal and crestal augmentation at Nos. 4 and 5 was performed using cancellous particulate allograft (Puros [Zimmer Biomet]), demineralized bone matrix (DMB) putty, and rh-PDGF-BB. The graft was covered with bilayered human pericardial membranes (CopiOs Pericardium Membrane [Zimmer Biomet]) (Figures 4 and 5). Passive primary closure was achieved using interrupted 4-0 expanded polytetrafluoroethylene (ePTFE) (GORE-TEX [W. L. Gore & Associates]) sutures.
Step 2: Implant placement of Nos. 4 and 5. After an 8-month healing period, a CBCT scan was taken, demonstrating a buccolingual dimension of 10 mm at ridge Nos. 4 and 5, a width gain of approximately 7 mm (Figure 6). The height of bone coronal to the maxillary sinus was 9 mm at site No. 4 (a vertical gain of 2.5 mm) and 17 mm at site No. 5 (a vertical gain of 4 mm).
Implants were placed at Nos. 4 and 5. To initiate the osteotomy, a pilot drill was used at 1,200 rpm with irrigation to a depth of 2 mm. Lekholm and Zarb type 3 bone density was noted. To expand the osteotomy sites and collect autogenous bone from them, a 2-mm twist drill was used at 75 rpm without irrigation to a depth of 10 mm. Both sites were further prepared with motorized osteotomes used at 75 rpm without irrigation to expand and densify the bone. Conventional implant drills were then used at 75 rpm without irrigation (OSSEOTITE Tapered Certain internal connection [Zimmer Biomet]) to final depths. During this slow-drilling process, autogenous bone was harvested from the osteotomies. At site No. 4, the maxillary sinus was elevated by 1 mm and augmented with cancellous particulate allograft bone mixed with rh-PDGF-BB.
At site No. 4, a 3.25- × 10-mm implant was placed with primary stability and a final torque of 90 Ncm (OSSEOTITE Tapered Certain). At site No. 5, a 3.25- × 10-mm implant was placed with primary stability and a final torque of 50 Ncm (OSSEOTITE Tapered Certain). Cover screws were placed. No dehiscence or fenestration defects were noted.
To enhance bone labial to the implants, encouraging a better aesthetic result, buccal GBR was performed using cancellous particulate autograft, autogenous bone, and rh-PDGF-BB overlaid with a resorbable collagen membrane (OsseoGuard [Zimmer Biomet]). Passive primary closure was achieved using a continuous 4-0 ePTFE suture.
Step 3: Second-stage implant exposure. Following a 3-month healing period, implant Nos. 4 and 5 were exposed via a palatally oriented crestal incision that allowed for buccal positioning of keratinized mucosa (Figure 7). The implants were integrated. Healing abutments (4 mm height, 4 mm diameter) were placed, and the mucosa was sutured with 4-0 polyglactin 910 (Vicryl Rapide [Ethicon]). A 5-mm band of keratinized mucosa labial to the abutments was achieved.
Step 4: Implant restoration. After one month of healing, the patient was referred to his restorative dentist. Individual screw-retained crowns were secured in at 32 Ncm (Figure 8). Complete hard- and soft-tissue regeneration was achieved.

Case 2: Cross-Arch Reconstruction
A nonsmoking 63-year-old female with an ASA II physical status presented with complications of 5 maxillary implants. Seven years prior to presentation at our practice, the patient had teeth Nos. 6 to 12 removed and the placement of immediate implants at Nos. 6 to 8, 10, and 11 with GBR by a previous surgeon. At second-stage surgery, the clinician had noted bone resorption around the implants that may have been related to ePTFE membrane exposure during healing. As the fixtures were integrated, the patient was restored with an implant- and tooth-supported, removable partial denture. Desiring a fixed prosthesis and improved cosmetics for the remaining maxillary teeth, the patient was referred to us by her general dentist.
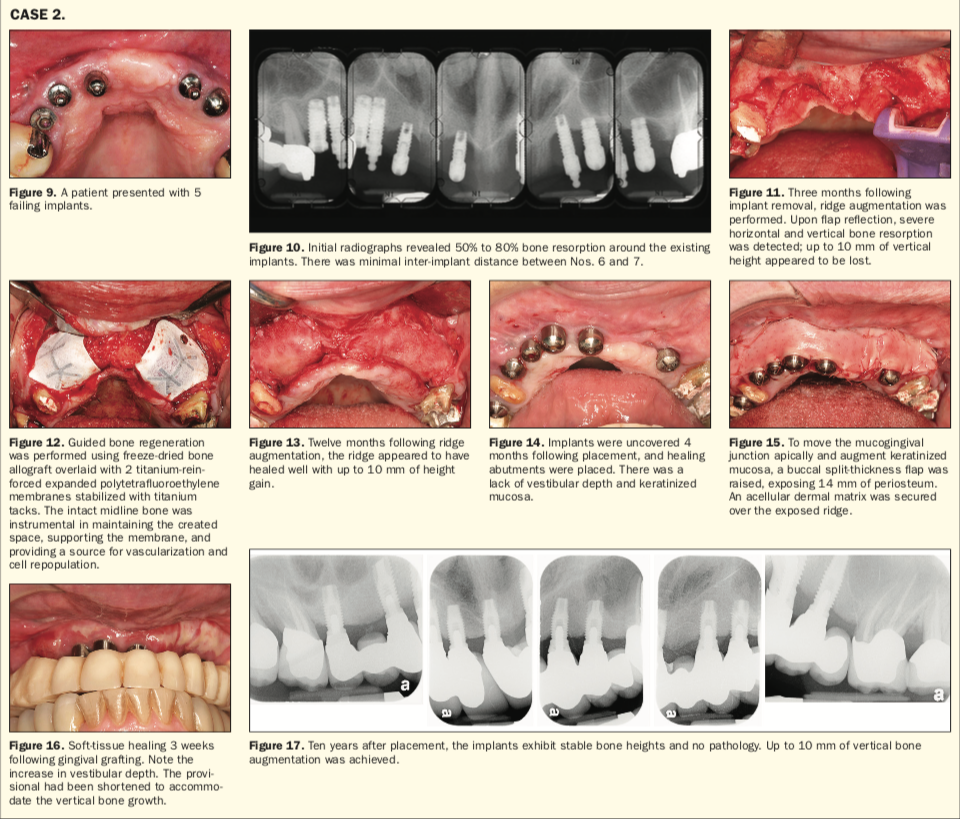
A clinical and radiographic examination was performed at our practice (Figures 9 and 10). Implant Nos. 8, 10, and 11 had more than 80% bone resorption but were nonmobile. The inter-implant distance between Nos. 6 and 7 was less than 1.5 mm. All implant sites exhibited mucogingival defects and suppuration.
The patient was diagnosed with peri-implantitis of Nos. 6 to 8, 10, and 11; generalized moderate periodontitis; caries on Nos. 3 and 5; and Miller Class III recession of maxillary teeth Nos. 3, 5, 14, and 15, all of which had crowns.
The initial treatment included full-mouth scaling and root planing and oral hygiene instructions.
For the maxillary arch, an ideal diagnostic wax-up was created to guide prosthetically driven treatment. The plan included the explantation of all implants with bone grafting in the residual sockets, provisionalization of the maxillary arch from Nos. 3 to 14, vertical and horizontal GBR for edentulous site Nos. 6 to 13, placement of 7 implants with additional GBR, second-stage exposure of implants, soft-tissue augmentation to enhance keratinized mucosa, and final restoration of Nos. 4 to 13 with an implant-retained FPD and new crowns for Nos. 3 and 14.
All surgery was performed under intravenous sedation, as administered by a dental anesthesiologist, and local anesthesia. One hour prior to each procedure, the patient took 500 mg amoxicillin and 600 mg ibuprofen. Post-op, the patient took ibuprofen 600 mg prn pain and amoxicillin 500 mg tid for 7 days. Unless stated otherwise, full-thickness buccal and palatal flaps were raised, extended sulcularly around adjacent teeth as needed, and periosteally released; vertical releases were placed as needed.
Step 1: Explantation of Nos. 6 to 8, 10, and 11. Elevation and forceps were used to remove implant Nos. 6 to 8, 10, and 11; minimal force was required. The implant sockets were thoroughly degranulated and grafted with freeze-dried bone allograft. Performed to create a healthy ridge and maintain soft-tissue volume prior to GBR, this procedure eliminated infection and provided some ridge support.
Step 2: Provisionalization of Nos. 3 to 14. An interim, metal-reinforced, acrylic, fixed partial denture #3-x-5-x-x-x-x-x-x-x-x-14 was fabricated to improve cosmetics and mastication and to alleviate pressure on the ridge during augmentation procedures.
Step 3: Vertical and horizontal GBR of Nos. 6 to 13. Three months after explantation, GBR was performed. An M-shaped vertical bone resorption pattern was present from Nos. 6 to 13, with a coronal projection of bone remaining at the midline that served as a buttress for augmentation; there appeared to be 9 to 10 mm of vertical resorption (Figure 11). Two titanium-reinforced ePTFE membranes were tacked apicobuccally. Following decortication, cancellous particulate allograft (Puros) was placed on the residual ridge from Nos. 6 to 13, supplementing it buccopalatally and apicocoronally. The membranes were positioned over the allograft, and their mobile ends were tucked beneath the palatal flap and fixed there with 4-0 ePTFE sutures (Figure 12). Passive primary closure was achieved with horizontal mattress and interrupted 4-0 polyglactin 910 (Vicryl Rapide) and 4-0 ePTFE (GORE-TEX) sutures.
Step 4: Implant placement at Nos. 4, 6 to 10, and 12. Twelve months after GBR, the ridge appeared to have been augmented horizontally by 7 mm and vertically by 6 to 10 mm (Figure 13). Osteotomy drilling was performed at 1,200 rpm with irrigation except at site Nos. 7 and 12, which were expanded with manual osteotomes. Lekholm and Zarb type 3 bone density was noted. Using a surgical guide, implants were placed at Nos. 4, 6 to 10, and 12 in accordance with the manufacturer’s protocol (OSSEOTITE Tapered External Hex Connection [Biomet 3i]). All implants placed were 4 × 10 mm except for No. 7, which was a 3.25- × 10-mm fixture. Primary stability was present. Cover screws were placed. No dehiscence or fenestration defects were noted.
To promote abundant bone labial to the implants, adjunct GBR was performed using cancellous particulate autograft and autogenous bone overlaid with a resorbable collagen membrane (OSSIX [Biomet 3i]). Passive primary closure was achieved using interrupted 4-0 polyglactin 910 and 4-0 ePTFE sutures.
Step 5: Second-stage exposure. Four months later, a full-thickness flap was elevated to uncover the implants, and healing abutments (4-mm height, 5-mm width) were placed (Figure 14). Implant No. 10 was nonintegrated and removed.
Minimal vestibular depths and keratinized mucosa deficits at implant Nos. 4, 6 to 9, and 12 were noted to be consistent with the magnitude of buccal flap mobilization at the time of vertical ridge augmentation. To correct these issues, soft-tissue augmentation with vestibular extension was scheduled.
Step 6: Soft-tissue augmentation. A mid-crestal incision was made at site Nos. 4 to 13, and a split-thickness buccal flap was raised. The flap margin was apically positioned by 14 mm and fixed to the periosteum with 5-0 chromic gut. Acellular dermal matrix was placed over the periosteum, secured to the mucosa via interrupted 4-0 polyglactin 910 sutures, and left exposed (Figure 15). Some shrinkage of the dermal matrix was expected.
Step 7: Restoration with an implant-retained FPD. After 2 months, 7 mm of buccal keratinized mucosa was present at implant Nos. 4, 6 to 9, and 12.
Tooth 5 was then extracted, and the patient’s prosthodontist restored the maxillary implants with an implant-retained FPD (#4-x-6-7-8-9-x-x-12-x). Teeth Nos. 3 and 14 were restored with porcelain-fused-to-metal crowns.
Ten years after treatment, the patient remains satisfied with function and aesthetics, and the implant bone and prosthesis are decidedly stable (Figures 16 and 17).
CONCLUSION
As these 2 cases demonstrate, successful implant rehabilitation in once-failed sites is attainable. A phasic surgical approach may be inevitable considering the level of tissue resorption present in implant failure. Because adjunct GBR was performed at implant placement, soft-tissue augmentation was deferred until after bone maturation; we theorize that a well-healed ridge better supports a mucosal graft and promotes its vascularization.12 Using expansion osteotomes or burs to preserve the buccolingual plates and elevate the maxillary sinus could also be beneficial.11,13,14 However, candidates for retreatment must be carefully screened; nonsmoking, medical and dental stability, and adherence to a strict maintenance regimen are musts.15
REFERENCES
1. Do TA, Le HS, Shen Y-W, et al. Risk factors related to late failure of dental implant—a systematic review of recent studies. Int J Environ Res Public Health. 2020;17(11):E3931. doi:10.3390/ijerph17113931
2. Chrcanovic BR, Albrektsson T, Wennerberg A. Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. Int J Prosthodont. 2017;30(3):219-237. doi:10.11607/ijp.5142
3. Schimmel M, Srinivasan M, McKenna G, et al. Effect of advanced age and/or systemic medical conditions on dental implant survival: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29 Suppl 16:311-330. doi:10.1111/clr.13288
4. Dreyer H, Grischke J, Tiede C, et al. Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res. 2018;53(5):657-681. doi:10.1111/jre.12562
5. Machtei EE. What do we do after an implant fails? A review of treatment alternatives for failed implants. Int J Periodontics Restorative Dent. 2013;33(4):e111-119. doi:10.11607/prd.1505
6. Oh S-L, Shiau HJ, Reynolds MA. Survival of dental implants at sites after implant failure: a systematic review. J Prosthet Dent. 2020;123(1):54-60. doi:10.1016/j.prosdent.2018.11.007
7. Gomes GH, Misawa MYO, Fernandes C, et al. A systematic review and meta-analysis of the survival rate of implants placed in previously failed sites. Braz Oral Res. 2018;32:e27. doi:10.1590/1807-3107bor-2018.vol32.0027
8. Zhou W, Wang F, Monje A, et al. Feasibility of dental implant replacement in failed sites: a systematic review. Int J Oral Maxillofac Implants. 2016;31(3):535-545. doi:10.11607/jomi.4312
9. Nassani MZ. What is the survival rate of dental implants placed at sites of failed implants? Evid Based Dent. 2019;20(3):95-96. doi:10.1038/s41432-019-0047-0
10. Brignardello-Petersen R. Implant replacement after 1 implant failure seems to have an acceptable rate of survival. J Am Dent Assoc. 2018;149(12):e164. doi:10.1016/j.adaj.2018.05.031
11. Sonick MK, Hwang D, Ma R. An atraumatic approach to internal sinus lifting: the motorized expansion drill technique. Compend Contin Educ Dent. 2020;41(6):331-335.
12. Mazzotti C, Stefanini M, Felice P, et al. Soft-tissue dehiscence coverage at peri-implant sites. Periodontology 2000. 2018;77(1):256-272. doi:10.1111/prd.12220
13. Padhye NM, Padhye AM, Bhatavadekar NB. Osseodensification—a systematic review and qualitative analysis of published literature. J Oral Biol Craniofac Res. 2020;10(1):375-380. doi:10.1016/j.jobcr.2019.10.002
14. Witek L, Neiva R, Alifarag A, et al. Absence of healing impairment in osteotomies prepared via osseodensification drilling. Int J Periodontics Restorative Dent. 2019;39(1):65-71. doi:10.11607/prd.3504
15. Roccuzzo M, Layton DM, Roccuzzo A, et al. Clinical outcomes of peri-implantitis treatment and supportive care: a systematic review. Clin Oral Implants Res. 2018;29 Suppl 16:331-350. doi:10.1111/clr.13287
ABOUT THE AUTHORS
Dr. Sonick is a world-recognized periodontal and dental implant surgeon with a doctorate from the University of Connecticut (UConn) School of Dental Medicine and a degree in periodontics from Emory University. He trained in implant dentistry at Gothenburg, Sweden’s Brånemark Clinic, and Harvard University. Having placed more than 10,000 successful implants, Dr. Sonick uses advanced technologies to regenerate bone and soft tissue, restoring patients to optimal health. He regularly teaches at New York University and UConn and he is the co-editor of Implant Site Development, a textbook on implant surgery. He can be reached at mike@sonickdmd.com.
Disclosure: Dr. Sonick has served as a consultant and educator for Zimmer Biomet.
Dr. Koo completed her Bachelor of Science degree at Brown University in neuroscience and subsequently received her DMD degree at the Harvard School of Dental Medicine. Dr. Koo pursued additional years of advanced training in periodontics at the UConn School of Dental Medicine, along with a masters in dental science. She is currently in private practice in Fairfield, Conn. She can be reached at stephanie@sonickdmd.com.
Disclosure: Dr. Koo reports no disclosures.
Dr. Ma is a fourth-generation dentist. He graduated summa cum laude with a Bachelor of Science in chemistry from the State University of New York at Albany, where he was class valedictorian. He earned his DMD degree from Tufts University and completed his postdoctoral training in periodontics at Stony Brook University, where he received a Certificate of Advanced Graduate Study in periodontics. He is currently in private practice in Fairfield, Conn. He can be reached at ray@sonickdmd.com.
Disclosure: Dr. Ma reports no disclosures.
Dr. Debby Hwang is a board-certified periodontist and implant surgeon. Dr. Hwang is the co-editor and author of the book Implant Site Development, an adjunct clinical assistant professor at the University of Michigan School of Dentistry, and a private practitioner in Ann Arbor, Mich. She received her doctorate from the Harvard School of Dental Medicine and her certificate in periodontics from the University of Michigan. She can be reached at debbyhwang@gmail.com.
Disclosure: Dr. Hwang reports no disclosures.











