INTRODUCTION
Bioactive refers to a material that causes an effect, or response, from living tissue. There are several new materials that are bioactive in that they stimulate dentin bridging and apatite formation by using calcium silicates or calcium aluminates.
TheraCal LC (BISCO Dental Products) is a liner, ProRoot MTA (DENTSPLY Tulsa Dental Specialties) is an endodontic reparative material, Biodentine (Septodont) is a base, and Ceramir (Doxa) is a cement; all of these materials have the potential to seal dentin, stop microleakage, almost eliminate sensitivity, and even promote pulpal healing.1 Today, instead of merely replacing tooth, we hope to stimulate the formation of tooth.
TheraCal, the focus of this article, is a material that creates a new category of resin-modified calcium silicates (RMCS). It is a light-cured, resin-based, and highly radiopaque liner designed to release calcium to promote hard-tissue formation, and is indicated for use under direct restorative materials as a replacement to calcium hydroxide (CaOH), glass ionomers, eugenol-based sedative materials, and pulp capping restoratives.
Liners and Pulp Capping Materials
Calcium silicates as found in mineral trioxide aggregate (MTA) have been used for many years as a bioactive material in various endodontic procedures as well as effective pulp capping and pulp replacement therapies.2,3 TheraCal LC consists of a single paste containing CaO, calcium silicate particles (type III Portland cement), Sr glass, fumed silica, barium sulphate, barium zirconate and resin containing Bis-GMA, and poly dimethacrylate. ProRoot MTA is composed of white Portland cement and bismuth oxide.4 While MTA has been shown to be a reliable pulp capping, dentin sealing material, and is biocompatible; its formulation and chemical curing make it a less efficient material to use routinely for restoration lining or pulp capping material.5
The rapid release of calcium in these materials makes it an excellent material to cause repairing and healing of dentin, and studies have shown it to be more effective at stimulating pulpal healing than traditional liners (such as light-cured resin-modified CaOHs) with less pulpal necrosis.6
The significant calcium release provides reparative ions, creates a sustaining alkaline environment required to promote wound healing, provides immediate bond and sealing properties, and stimulates hydroxyl-apatite and secondary dentin formation within affected tissues.
Theracal LC has been approved as “apatite stimulating” by the US Food and Drug Administration and is an interactive flowable resin that provides the early high alkalinity, pH 10 to 11, required for pulpal healing but reverts back to a neutral pH after several days.7 It is self-sealing, which aids in antimicrobial activity with initial bonds to dentin to resist accidental air-drying removal. This high calcium release has been shown to be critical for the stimulation of apatite formation and secondary dentin bridge formation while providing a mechanical seal of the pulp without adhesive.8
Dentists have been placing liners under restorations for many years as a pulp protectant to reduce pulpal necrosis and sensitivity.9 When pulpal tissue is exposed during tooth preparation, certain liners have been shown to reduce pulpal infection and necrosis than direct bonding onto the pulp alone.10 Direct pulp capping with some self-etching adhesive systems have shown dramatic histologic failure indicating the need for a bioactive liner to stimulate reparative dentin formation.11
CaOH has been used for decades in hopes of stimulating reparative dentin formation by its high alkalinity. This liner may weaken the restoration, cause negative effects with restoration longevity, and may cause a decrease in bonding strengths of the restoration to the tooth.12 As a pulp capping material, CaOH has had mixed results that have diminished with time after placement.13 Newer resin-based materials based on the MTA and other high calcium releasing materials have superior long-term sealing ability and they have the potential for greater stimulation of reparative dentin.14
A major drawback of traditional self-cured CaOH materials is high solubility and dissolution over time (within one to 2 years after application) in tissue fluids. Fluids from restoration leakage, dentinal tubules, or the pulp may cause the disappearance of this type of lining material and the formation of voids/defects in reparative dentine underneath the capping.15 This can lead to a failure of the definitive seal against bacterial invasion and restoration failure.16 By contrast, the RMCS liner, TheraCal LC, has a very low solubility leaving a secure lining despite contact with dentinal or pulpal fluids; at the same time it releases substantial amounts of calcium for apatite stimulation.17
Clinical uses for this new RMCS include: as a general liner under restorations before etching or dentinal bonding, as a liner for pulp protection in restorations placed close to the pulp, as a liner over remaining pulp in deciduous pulpotomies or pulpectomies, and as a pulp capping material in permanent or deciduous teeth.
Light-curing of the liner is a large clinical benefit. Clinically, it must be remembered that TheraCal LC is a liner only, and the manufacturer recommended depth-of-cure of about one mm means it must be placed in thinner increments than a more “base-like” material. Light-curing as opposed to chemical curing makes this material an excellent choice for office efficiency as definitive restorations can be placed immediately and the setting of the material is predictable even in the moist environment of the pulp. Because this material is opaque and “whitish” in color, it should be kept thin so as to not show through composite materials that are very translucent or very shallow affecting final restoration shading.
Direct Pulp Capping
Direct pulp capping is the treatment of the exposed vital pulp by sealing the pulpal wound with a dental material to induce a reparative dentinogenic response, and is one of the most important endodontic modalities for maintaining dental pulp vitality.18,19 The material must act as a barrier protecting the pulp while inducing the formation of new dentine bridge between the pulp and restorative material.15
The bioavailability of calcium (Ca) ions plays a key role in this process by stimulating cells involved in the new formation of mineralized hard tissues.20 Ca ions stimulate the expression of bone-associated proteins mediated by calcium channels and large quantities of Ca ions could activate adenosine triphosphate, which plays a significant role in the mineralization process.21 Ca ions are also necessary for the differentiation and mineralization of pulp cells and formation of odontoblastic cells which are needed to formulate the apatite of the dentin bridge.22 This release of calcium at the pulp also enhances the activity of pyrophosphatase, which helps to maintain dentin mineralization and the formation of new dentin bridging.23
Preliminary evidence on the formation of apatite on TheraCal LC (as well as ProRoot MTA and Dycal) has been obtained, showing that the phosphate ions of biological fluids such as blood, plasma, and dentinal fluid may react with Ca and OH ions leached from these materials and cause apatite crystal precipitation.24-26 This is the probable mechanism of action and is the process bioactive liners try to accomplish.
There are several factors leading to successful pulp capping, including the age of the patient, the control of bleeding, the size of the exposure, infection of the pulp, length of pulp violation, and others.9,27,28 There have been many materials used to stop bleeding and site preparation directly over the pulp such as sodium hypochlorite, formocresol, ferric sulfate, saline, aluminum chloride, superoxol, and others.29 Some materials such as ferric sulfate and formocresol may negatively influence the ultimate bond strength of the restoration, possibly leading to bacterial leakage that may cause a failed pulp capping.30
CASE REPORT
Diagnosis and Treatment Planning
A 16-year-old male broke tooth No. 9 off at the incisal portion of the pulp while lifting weights at school (Figures 1 and 2). The broken piece of tooth was placed in milk (2%) and was brought to the office about 45 minutes after the accident. The break was at a level where about two thirds of the tooth was missing and the dentition otherwise was intact with good oral hygiene (Figure 3). The pulp was clean with very little bleeding (Figure 4).31
 |
 |
| Figure 1. Traumatic fracture of incisal two thirds of tooth No. 9. | Figure 2. The tooth was brought to the office in milk within one hour of the accident. |
 |
 |
| Figure 3. The fracture was horizontal with no other signs of damage other than a small horizontal craze line in the gingival portion of tooth No. 8. | Figure 4. Obvious pulpal exposure with little bleeding. |
 |
 |
| Figure 5. Radiograph showed no other obvious fractures or problems. | Figure 6. Because of the relatively short time of exposure, the bleeding control, and availability of an apatite/dentin stimulating product, a direct pulp cap and composite was planned. |
 |
 |
| Figure 7. The tooth fragment was in good shape with a slight fracture in the incisal edge. | Figure 8. The pulp was disinfected and bleeding controlled with sodium hypochlorite for 30 seconds, then rinsed. |
 |
 |
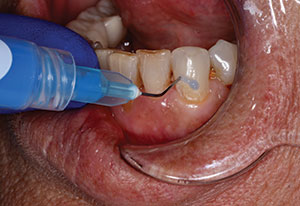
| Figure 9. A 1-mm thick layer of a resin-modified calcium silicate (RMCS) liner (TheraCal LC [BISCO Dental Products]) was placed over the pulp and light-cured from 3 different directions for 10 seconds each. | Figure 10. The liner was placed 1 mm onto dentin beyond the exposure, keeping it away from enamel. |
Because of the relatively short time of exposure, the controlled bleeding, and the overall general health of the patient, a successful result was expected. However, the patient was informed of the possibility of darkening, sensitivity, periapical radiolucency, spontaneous pain, swelling, and other symptoms that may indicate the need for subsequent endodontic treatment.32 Radiographic examination showed no obvious subgingival fractures or PA problems (Figure 5).
After warning the patient about signs of infection or necrosis, vital pulp capping with a RMCS liner (TheraCal LC) was agreed upon (Figure 6).
Clinical Protocol
The moist tooth fragment was inspected, rinsed, and placed on the tooth to verify the fit (Figure 7). Only a small amount of incisal edge was missing.
The surface of the broken tooth and pulp was cleaned with sodium hypochlorite on a microbrush and allowed to set for about 30 seconds (Figure 8). A one-mm layer of TheraCal LC was then placed and light-cured 10 seconds from the occlusal, 10 seconds from the facial, and 10 seconds from the lingual to insure adequate polymerization (Figure 9). Care was taken to place the liner 0.5 mm to 1.0 mm onto sound dentin away from the defect while keeping the material away from the enamel so that excellent sealing of the restoration could occur (Figure 10).
The moist tooth fragment was hollowed out with a 330 bur to provide relief where the liner was placed (Figure 11). After verification of fit it was then etched with phosphoric acid (Etch 37 with BAC [BISCO Dental Products]) for 20 seconds and rinsed thoroughly (Figure 12). The benzylkonium chloride, benzalkonium chloride (BAC), is a microbial agent that aids in the killing of bacteria and may help with the disinfection of the preparation. After rinsing, several coats of a universal dentin bonding agent was applied (All Bond Universal [BISCO Dental Products]) and air-thinned (Figure 13).
 |
 |
| Figure 11. A 330 bur was used to remove about one mm of tooth structure on the fragment to compensate for the thickness of the RMCS liner. The piece was tried in the mouth to ensure complete seating. | Figure 12. The piece was etched with BAC containing 37% phosphoric acid (BISCO Dental Products) and then rinsed thoroughly. |
 |
 |
| Figure 13. A universal bonding agent, All Bond Universal (BISCO Dental Products), was placed in several coats and air thinned. | Figure 14. The remaining tooth was isolated with retractors, a Mylar strip placed, and tooth-etched for 10 seconds. |
 |
 |
| Figure 15. The same universal bonding agent was applied to the tooth and air-thinned. | Figure 16. A layer of giomer flowable composite resin (Beautifil Flow Plus [Shofu Dental]) was placed on the defect side of the fragment and placed on the tooth. |
 |
 |
| Figure 17. The flash of extra material was left in place and cured so as not to disturb the bonding of the material, and then light-cured. | Figure 18. After polymerization, the flash was removed along with about 1 mm of facial enamel so that a composite veneer could be placed to add strength to the restoration. |
 |
 |
| Figure 19. After preparation, the facial surface was etched, bonded, and composite was placed. Characterization was done with a composite resin stain. | Figure 20. The restoration was shaped, polished, and taken out of occlusion. |
Inside the mouth the tooth was isolated with retractors (See-More [Phillips]), a Mylar strip placed, and phosphoric acid with the BAC etchant placed, rinsed thoroughly, and suctioned moist (Figure 14). Several coats of universal bonding agent (All Bond Universal) were applied, air thinned, and light-cured for 20 seconds (Figure 15). A giomer flowable composite (Beautifil Flow Plus [Shofu Dental]) was placed inside the broken piece and repositioned onto the tooth (Figure 16). This material has a great combination of strength, flow, and fluoride release and rechargeability, making it ideal for proper fragment positioning and inhibition of microleakage.33,34 Light-curing was then done for 20 seconds from both the lingual and facial (Figure 17). The flowable composite was allowed to flow out from the restored piece and cured with flash intact so that the fragment would not be “bumped,” disturbing the bonding of the material (Figure 18).
To increase the overall strength of the restoration and decrease the chances of microleakage, the facial was veneered with a microhybrid composite. After thorough curing, the flowable flash that remained from fragment placement was removed with a diamond bur and the enamel was roughened removing around one mm of tooth structure. The facial was then etched and dentin bonding agent applied. Tints and layers of composite were added to mimic the aesthetic characteristics of the tooth and restoration of the incisal chip (Figure 19). Shaping was done with a finish diamond, bur, and polishing disks (Figure 20). The tooth was adjusted out of occlusion slightly. The opacity of the RMCS liner is readily evident in the radiograph (Figure 21).
 |
 |
| Figure 21. TheraCal LC is very radiopaque, making it easily identifiable. | Figure 22. The result is a conservative restoration that is well-sealed. |
 |
 |
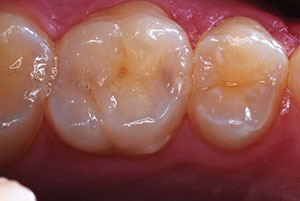
| Figure 23. At 10 months, the restoration and vitality of the tooth remain with a pleasing outcome. | Figure 24. Should symptoms arise and endodontic therapy be needed, a ceramic veneer or full-coverage all-ceramic crown could be considered and done. |
CLOSING COMMENTS
The result was an aesthetically pleasing and minimally invasive restoration (Figures 22 and 23). The preoperative condition of the tooth (fractured and involving the pulp) presented a wide range of therapeutic options to the dentist. The mantra of conservative dentistry is carried on as long as the therapy provided has a reasonable degree of expected success and the patient is warned of signs of failing treatment; and in this case, this included the possibility of darkening, pain, swelling, and radiographic signs of necrosis. At 10 months, the restoration is asymptomatic and the restoration is functioning well (Figure 24). The patient is also fully aware of the need for further restorative work in the future.
References
- Colon P, Bronnec F, Grosgogeat B, et al. Interactions between a calcium silicate cement (Biodentine) and its environment. J Dent Res. 2010;89. Abstract 401.
- Dammaschke T, Wolff P, Sagheri D, et al. Mineral trioxide aggregate for direct pulp capping: a histologic comparison with calcium hydroxide in rat molars. Quintessence Int. 2010;41:20-30.
- Leye Benoist F, Gaye Ndiaye F, Kane AW, et al. Evaluation of mineral trioxide aggregate (MTA) versus calcium hydroxide cement (Dycal) in the formation of a dentine bridge: a randomised controlled trial. Int Dent J. 2012;62:33-39.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—Part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400-413.
- Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review—Part II: leakage and biocompatibility investigations. J Endod. 2010;36:190-202.
- Dickens SH, Flaim GM, Schumacher GE, et al. Preclinical effectiveness of a novel pulp capping material. J Endod. 2010;36:1222-1225.
- Gandolfi MG, Suh B, Taddei P, et al. Chemical-physical properties of TheraCal pulp-capping material. Poster presented at: 89th General Session & Exhibition of the IADR; March 18, 2011; San Diego, CA. Abstract 2521.
- Gandolfi MG, Siboni F, Taddei P, et al. Apatite-forming ability of TheraCal pulp-capping material. Poster presented at: 89th General Session & Exhibition of the IADR; March 18, 2011; San Diego, CA. Abstract 2520.
- Aguilar P, Linsuwanont P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: a systematic review. J Endod. 2011;37:581-587.
- Dammaschke T, Stratmann U, Fischer RJ, et al. A histologic investigation of direct pulp capping in rodents with dentin adhesives and calcium hydroxide. Quintessence Int. 2010;41:e62-e71.
- da Silva LA, de Freitas AC, de Carvalho FK, et al. Direct pulp capping with a self-etching adhesive system: histopathologic evaluation in dogs’ teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e34-e40.
- Wang Y, Liao Z, Liu D, et al. 3D-fEA of stress levels and distributions for different bases under a Class I composite restoration. Am J Dent. 2011;24:3-7.
- Willershausen B, Willershausen I, Ross A, et al. Retrospective study on direct pulp capping with calcium hydroxide. Quintessence Int. 2011;42:165-171.
- Witherspoon DE. Vital pulp therapy with new materials: new directions and treatment perspectives—permanent teeth. Pediatr Dent. 2008;30:220-224.
- Desai S, Chandler N. Calcium hydroxide-based root canal sealers: a review. J Endod. 2009;35:475-480.
- Hørsted-Bindslev P, Løvschall H. Treatment outcome of vital pulp treatment. Endodontic Topics. 2002;2:24-34.
- Gandolfi MG, Siboni F, Prati C. Chemical-physical properties of TheraCal, a novel light-curable MTA-like material for pulp capping. Int Endod J. 2012;45:571-579.
- Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13-27.
- Modena KC, Casas-Apayco LC, Atta MT, et al. Cytotoxicity and biocompatibility of direct and indirect pulp capping materials. J Appl Oral Sci. 2009;17:544-554.
- Jung GY, Park YJ, Han JS. Effects of HA released calcium ion on osteoblast differentiation. J Mater Sci Mater Med. 2010;21:1649-1654.
- Torneck CD, Moe H, Howley TP. The effect of calcium hydroxide on porcine pulp fibroblasts in vitro. J Endod. 1983;9:131-136.
- Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985;64 Spec No:541-548.
- Estrela C, Holland R. Calcium hydroxide: study based on scientific evidences. J Appl Oral Sci. 2003;11:269-282.
- Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127-1137.
- Gandolfi MG, Taddei P, Tinti A, et al. Apatite-forming ability (bioactivity) of ProRoot MTA. Int Endod J. 2010;43:917-929.
- Gandolfi MG, Van Landuyt K, Taddei P, et al. Environmental scanning electron microscopy connected with energy dispersive x-ray analysis and Raman techniques to study ProRoot mineral trioxide aggregate and calcium silicate cements in wet conditions and in real time. J Endod. 2010;36:851-857.
- Ward J. Vital pulp therapy in cariously exposed permanent teeth and its limitations. Aust Endod J. 2002;28:29-37.
- Al-Hiyasat AS, Barrieshi-Nusair KM, Al-Omari MA. The radiographic outcomes of direct pulp-capping procedures performed by dental students: a retrospective study. J Am Dent Assoc. 2006;137:1699-1705.
- Odabaş ME, Cinar C, Tulunoğlu O, et al. A new haemostatic agent’s effect on the success of calcium hydroxide pulpotomy in primary molars. Pediatr Dent. 2011;33:529-534.
- Shalan H, Awad S, El-Fallal AA. Influence of pulpotomy medicaments on the ultrastructure and shear bond strength of a self-etch adhesive to primary tooth dentin. Quintessence Int. 2012;43:517-523.
- Emine ST, Tuba UA. White mineral trioxide aggregate pulpotomies: Two case reports with long-term follow-up. Contemp Clin Dent. 2011;2:381-384.
- Witherspoon DE, Small JC, Harris GZ. Mineral trioxide aggregate pulpotomies: a case series outcomes assessment. J Am Dent Assoc. 2006;137:610-618.
- Naoum S, Ellakwa A, Martin F, et al. Fluoride release, recharge and mechanical property stability of various fluoride-containing resin composites. Oper Dent. 2011;36:422-432.
- Gordan VV, Mondragon E, Watson RE, et al. A clinical evaluation of a self-etching primer and a giomer restorative material: results at eight years. J Am Dent Assoc. 2007;138:621-627.
Dr. Griffin graduated in 1988 from Southern Illinois University and then went on to complete a general practice residency at the University of Louisville in Kentucky. He owns and operates a general practice in Eureka, Mo. Dr. Griffin on current clinical topics including CAD/CAM-digital dentistry, adhesion, photography, and practice management. He can be reached at esmilecenter@aol.com, or via the Web site eurekasmile.com.
Disclosure: Dr. Griffin reports no disclosures.