Fluoride’s ability to inhibit or even reverse the initiation and progression of dental caries is well documented. Fluoride varnishes were developed to improve the efficacy and safety of topical fluoride. For more than 30 years, fluoride varnishes have been the standard of care for the professional application of topical fluoride in Europe.1 The primary reasons for the wide acceptance of fluoride varnishes include the ease of use, safety, and convenient application procedure.2 With fluoride varnishes, the amount of fluoride exposure to patients can be better controlled, and less chair time is required compared with the conventional use of foams and gels that require suction devices and trays. The effectiveness and safety of fluoride varnishes are documented in more than 50 clinical trials.3
The use of fluoride varnish for caries prevention has increased among the dental community in the United States since its introduction in the 1990s.4 The purpose of this article is to review the recent research findings and the efficacy and safety of fluoride varnish, review the products available in the United States, and suggest guidelines for the use of fluoride varnish for caries control. A PubMed search was conducted with keywords “fluoride varnish” starting in year 1985 to current, limiting the search to “reviews.” Further, the Cochrane Database of Systemic Reviews was examined.
EFFICACY OF FLUORIDE VARNISHES
Fluoride varnishes were originally developed to prolong the contact time between fluoride and the tooth surfaces, thereby improving fluoride incorporation into the surface layers of the tooth, ie, uptake of fluoride that is firmly bound to enamel.2 However, the concept of the cariostatic mechanism of fluoride has changed considerably over the past decades. In addition to fluoride incorporation into the crystalline lattice, fluoride varnishes interact with saliva and form calcium fluoride (CaF2) compounds on enamel.2,5 These calcium fluoride deposits create a reservoir of fluoride ions, which are slowly released when the pH of plaque drops, thus acting as a prolonged source of fluoride ion.6 This has been considered the most important action mechanism of the products with high fluoride concentrations. It has been noticed that fluoride varnishes are effective when used on early white spot lesions, since a large amount of fluoride can be deposited in the porous demineralized enamel. Thus, the action of fluoride can be related to its inhibition of the demineralization processes as well as its promotion of enamel remineralization.
In numerous studies, fluoride varnishes have been shown to be clinically effective in preventing caries.3,7-10 However, studies vary widely in their design and in the rate of caries reduction. Studies conducted between 1968 and 1985 reported an overall reduction in caries increment ranging from 18% to 77%.7 According to the Cochrane Review by Marinho, et al9, the application of fluoride varnishes 2 to 4 times a year, either in the permanent or the deciduous dentition, is associated with a substantial reduction in the caries increment.
Duraphat (Colgate Oral Pharmaceuticals) varnish has been the most extensively studied fluoride varnish, producing caries reductions in both primary and permanent dentition.3,7-10 Several reviews report evidence of the efficacy of Duraphat, and recommend its use for caries control.3,7-12 The National Institutes of Health Consensus Development Conference on Diagnosis and Caries Management,13 the Centers for Disease Control and Prevention,14 and American Dental Association Council on Scientific Affairs12 support the beneficial effect of fluoride varnish on the permanent teeth and recommend the use of fluoride varnish for children and adults at moderate or high risk for caries as professionally applied topical fluoride, in addition to toothbrushing twice a day with fluoride toothpaste. However, since the majority of fluoride varnish studies have been conducted in children and adolescents, there is still a need for further studies on the effect of fluoride varnishes in the elderly, especially those with root caries.13
FLUORIDE VARNISHES AVAILABLE IN THE UNITED STATES
Several fluoride varnishes are currently available in the United States: Duraphat (Colgate Oral Pharmaceuticals), Duraflor (Pharmascience), CavityShield (OMNI Preventive Care, a 3M ESPE dental company), and Fluor Protector (Ivoclar Vivadent). Recently, several new products have been introduced to the market, such as Fluoridex Lasting Defense (NaF) Varnish (Discus Dental), VarnishAmerica (Medical Products Laboratories), DuraShield (Sultan Dental Products), AllSolutions (DENTSPLY), Vanish (OMNI Preventive Care), Colgate-Prevident (Colgate Oral Pharmaceuticals), and Flor-Opal Varnish (Ultradent Products). All of these varnishes, except Fluor Protector, contain 5% sodium fluoride (22,600 parts per million fluoride ions [F-]). Duraphat and Duraflor are packaged in a 10 ml tube, and the others are packaged individually for single-unit dose applications. CavityShield and VarnishAmerica are packaged in 0.25 ml and 0.4 ml doses for single use, and DuraShield, Vanish, and ColgatePrevident are packaged in 0.4 ml doses. Fluor-Protector contains 0.9% difluorosilane by weight (1,000 ppm F-) in polyurethane-based varnish, and sets to a thin transparent film. It comes in either a 0.4 ml vial for single-use or a 1.0 ml ampule for multiple doses. ColgatePrevident dries to a transparent enamel color, and Vanish has a white color. Flor-Opal Varnish has a unique syringe-to-syringe mixing and delivery system that eliminates separation of fluoride from the resin carrier, along with a bendable tip.
An in vivo study by Shen and Autio-Gold15 compared the uniformity of the fluoride concentration in different types of varnishes—Duraphat, Duraflor, CavityShield, and Fluor Protector. The intent was to evaluate possible ingredient separation.15 When doses from these varnish tubes were compared, Duraphat showed more uniformity and less separation of ingredients than Duraflor.15 According to the manufacturer, the individual dose units of CavityShield are intended to be mixed by hand in the provided well, which could reduce separation and thus improve uniformity. The separation problem should be kept in mind when considering other single-dose unit systems, and mixing could be indicated before clinical application. Some of these varnishes also appear as a light, yellowish-brown layer on the teeth surfaces after application. However, this discoloration is not permanent and disappears after a day or 2 with regular toothbrushing.16 For patients who do not want this light yellow color on the day of application, white varnishes could be used, such as Vanish and ColgatePrevident.
In 1994, the United States Food and Drug Administration (FDA) approved Duraphat for marketing as a medical device to be used as a cavity liner and for the treatment of hypersensitive teeth. Because caries prevention is considered a drug claim, manufacturers would have to submit appropriate clinical trial evidence for review by the FDA before they could be cleared as anticaries agents.3 In the United States, the therapeutic use of fluoride varnishes for caries prevention is referred to as “off-label” because the product is being used for purposes other than originally approved, and they can be used for caries prevention in clinical practice.17 In the states of Washington and North Carolina, treatment with fluoride varnishes is a preventive service procedure covered by Medicaid.18 Several states, such as Alaska, Idaho, Iowa, Georgia, North Carolina, Kansas, Nevada, Virginia, and Washington, have developed prevention programs, manuals, and billing procedures for fluoride varnish to be used as a caries preventive agent.4
Fluoride varnishes marketed in the United States have the highest fluoride concentration of any fluoride vehicle (22,600 ppm F-), and some ingestion of the fluoride can occur during the application process or after fluoride is released into the saliva. However, there are no reports of possible side effects or adverse effects for patients.11 Ekstrand, et al19 evaluated the plasma fluoride concentration and urinary fluoride excretion following applicaton of Duraphat varnish. Their studies revealed that urinary fluoride concentration 12 hours after application was between 500 and 1,100 µg F-, which is well below the toxic level. The comprehensive review to determine the safety of fluoride varnish for the Cochrane Collaboration database found no information about adverse effects in the clinical trials that were reviewed.9 However, it was suggested that future studies collect additional data on possible side effects. Among clinicians, fluoride varnish applications have been regarded as safe even for young children, since the amount of varnish is usually less than 0.5 ml, which delivers 3 to 11 mg of fluoride ion, far below the probable toxic dose of 5 mg/kg.
CLINICAL CONSIDERATIONS
Frequency of Application
For a fluoride varnish to be effective, frequency of fluoride varnish applications should be based on an individual caries risk assessment.4 The most frequently prescribed regimen has been a semiannual application of varnish.20 Petersson and Westerberg21 suggested that 3 applications of varnish in one week, conducted on an annual basis, could be more effective than seminannual application. However, this application frequency requires further study in order to be established as a standard recommendation. For high and moderate risk individuals, varnish could be recommended to be applied 2 to 4 times a year.9,12
Indications
 |
|
Figure 1. Early, noncavitated enamel lesions, ie, white-spot lesions, which have potential to remineralize with the use of fluoride varnish. |
Fluoride varnishes are recommended for patients with a high or moderate risk of caries.4 To assess a patient’s caries risk, several risk factors can be identified through the use of clinical and sociodemographic information, which is routinely collected at annual clinical examinations. Several protocols have been developed for risk assessment. The National Institutes of Health13 published recommendations for the most helpful and consistent risk indicators in practice, which are: (1) past caries experience, (2) inadequate previous or current exposure to fluoride, (3) any physical or mental illness and any oral appliance or restoration that compromises the maintenance of optimal oral health, (4) frequent fermentable carbohydrate consumption, (5) lower salivary flow, associated with certain medical conditions and therapies, (6) high mutans streptococci levels, (7) gingival recession, especially in elderly populations, and (8) lower index of socioeconomic status.
Based on clinical findings, patients with a high caries risk with active noncavitated lesions (Figure 1) and exposed root surfaces can benefit from fluoride varnish. After periods of tooth eruption, when enamel is still not fully mineralized, patients can benefit from the mineralizing effect of fluoride varnish and the greater uptake of fluoride. Patients with reduced salivary flow, or following periodontal surgery, and patients with fixed or removable prostheses can temporarily have higher risk for decay and also benefit from fluoride varnish applications. Individuals with an eating disorder, or mentally or physically challenged individuals can also have a high caries risk. Varnish has been regarded as a safe and easy alternative for caries control in patients with special needs, such as those receiving head and neck radiation, orthodontic treatment, and those using medications that result in reduced salivary flow.18 As suction devices and trays are not needed for fluoride varnish application, varnish can be applied even for very young children and in field situations, such as the classroom.22 It has been suggested that fluoride varnish can be adopted into medical practice, applied by primary care physicians and their staff.23
Clinically, varnish can be applied to fissures, proximal surfaces, or smooth surfaces of primary or permanent teeth. It can also be targeted only to specific tooth surfaces, and applications can be done according to individual needs. Varnish should be applied to dry, clean teeth. However, professional prophylaxis of the teeth is not essential before application. It has been shown that fluoride ions can migrate through plaque, and toothbrushing performed by the patients themselves is sufficient prior to varnish application.24
Clinical Application
 |
|
Figure 2. Teeth isolated with cotton rolls and dried with compressed air or with cotton gauze before fluoride varnish application. |
The following steps are recommended for the clinical application of fluoride varnish:
1. Isolate the quadrant with cotton rolls, and dry with compressed air or with cotton gauze before application (Figure 2). Since varnish sets in the presence of moisture, excessive drying is not necessary.
 |
|
Figure 3. When a single unit dose system is used, mix fluoride varnish in a well before application. |
2. When a single-dose system is used, mix the varnish in the well that is provided (Figure 3). For the adult dentition, 0.4 ml of varnish is adequate. Due to the high concentration of fluoride, care should be taken not to exceed the recommended dose.
 |
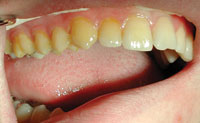 |
|
Figure 4. Apply fluoride varnish onto the teeth surfaces with a small disposable brush. |
Figure 5. Varnish should be applied as a thin film. |
3. Apply varnish on the dried teeth surfaces or specific tooth surface with a small disposable brush (Figure 4) or with the brush provided in the single-dose unit. Varnish should be applied as a thin film (Figure 5). A specific setting time is not required since varnish sets in contact with saliva. After the application, cotton rolls can be removed and the clinician can proceed to the next quadrant. The application process usually takes one to 4 minutes.
4. Instruct patients to avoid eating for 2 to 4 hours after the application and to eat a soft diet for the rest of the day. Patients should avoid brushing the same day to maximize contact between the varnish and teeth, and to achieve optimal fluoride benefit. The varnish can be brushed away with normal tooth brushing the next day. Patients should also be told about the temporary yellowish coating of the teeth when Duraphat, CavityShield, Duraflor, or similar products are used. In vivo testing has shown that Duraphat, Cavity-Shield, and Duraflor can be used without adversely affecting the hue and value (ie, the color) of aesthetic restorative materials.16 The fee for the application of fluoride varnish can be charged as a “topical fluoride application.” It has been estimated that the application costs are $1 to $4 depending on the brand used. The major expense is the time and related personnel costs required to apply the varnish.11
SUMMARY
Available data suggest that fluoride varnish can be a safe and effective method for caries management. The application of varnish can be beneficial for those at risk for caries and for patients with special needs, and for those with no access to daily fluoride or other preventive methods. Even a small amount of varnish can be applied to active noncavitated lesions, assuring that a high concentration of the agent is available at the site where needed and that the total amount of active agent administered to the patient may be markedly reduced. Considering that varnish treatment is painless and can be easily performed by auxiliary dental personnel, it is a caries preventive method that can be easily applied and recommended for any age group, even young children. For high-risk caries patients with a significant cariogenic challenge, topical applications of fluoride might be insufficient and thus could be supplemented with other anticariogenic methods, such as xylitol chewing gum.25
References
- Bawden JW. Fluoride varnish: a useful new tool for public health dentistry. J Public Health Dent. 1998;58:266-269.
- Øgard B, Seppä L, Rølla G. Professional topical fluoride applications – clinical efficacy and mechanism of action. Adv Dent Res. 1994;8:190-201.
- Beltran-Aguilar ED, Goldstein JW, Lockwood SA. Fluoride varnishes. A review of their clinical use, cariostatic mechanism, efficacy and safety. J Am Dent Assoc. 2000;131:589-596.
- Autio-Gold J. Fluoride varnishes for everyday practice. Pract Proced Aesthet Dent. 2005:17:398, 400.
- Fejerskov O, Thylstrup A, Larsen MJ. Rational use of fluorides in caries prevention. A concept based on possible cariostatic mechanisms. Acta Odontol Scand. 1981;39:241-249.
- Ten Cate JM. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur J Oral Sci. 1997;105(5 pt 2):461-465.
- de Bruyn H, Arends J. Fluoride varnishes: a review. J Biol Buccale. 1987;15:71-82.
- Petersson LG. Fluoride mouthrinses and fluoride varnishes. Caries Res. 1993;27(suppl 1):35-42.
- Marinho VC, Higgins JP, Logan S, et al. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2002;(3):CD002279.
- Seppä L. Fluoride varnishes in caries prevention. Med Princ Pract. 2004;13:307-311.
- Weintraub JA, Hysan L. Fluoride varnish for caries prevention: comparison with other preventive agents and recommendations for a community-based protocol. Special Care Dentistry. 2003;23:180-186.
- American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc. 2006;137:1151-1159. http://www.ada.org/prof/resources/pubs/jada/reports/report_fluoride.pdf. Accessed November 26, 2007.
- National Institutes of Health (US). Diagnosis and management of dental caries throughout life. NIH Consens Statement. 2001;18(1):1-23.
- Recommendations for using fluoride to prevent and control dental caries in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2001;50(RR-14):1-42.
- Shen C, Autio-Gold J. Assessing fluoride concentration uniformity and fluoride release from three varnishes. J Am Dent Assoc. 2002;133:176-182.
- Autio-Gold JT, Barrett AA. Effect of fluoride varnishes on color stability of esthetic restorative materials. Oper Dent. 2004;29:636-641.
- US Food and Drug Administration. The FDA Modernization Act of 1997. FDA Web site. http://www.fda.gov/opacom/backgrounders/modact.htm. Published November 21, 1997. Accessed November 26, 2007.
- Vaikuntam J. Fluoride varnishes: should we be using them? Pediatr Dent. 2000;22:513-516.
- Ekstrand J, Koch G, Petersson LG. Plasma fluoride concentration and urinary fluoride excretion in children following application of the fluoride-containing varnish Duraphat. Caries Res. 1980;14:185-189.
- Hawkins R, Locker D, Noble J, et al. Prevention. Part 7: professionally applied topical fluorides for caries prevention. Br Dent J. 2003;195:313-317.
- Petersson LG, Westerberg I. Intensive fluoride varnish program in Swedish adolescents: economic assessment of a 7-year follow-up study on proximal caries incidence. Caries Res. 1994;28:59-63.
- Autio-Gold JT, Courts F. Assessing the effect of fluoride varnish on early enamel carious lesions in the primary dentition. J Am Dent Assoc. 2001;132:1247-1253.
- Lewis C, Lynch H, Richardson L. Fluoride varnish use in primary care: what do providers think? Pediatrics. 2005;115:69-76.
- Seppä L. Effect of dental plaque on fluoride uptake by enamel from a sodium fluoride varnish in vivo. Caries Res. 1983;17:71-75.
- Hayes C. The effect of non-cariogenic sweeteners on the prevention of dental caries: a review of the evidence. J Dent Educ. 2001;65:1106-1109.
Dr. Autio-Gold received her DDS and PhD degrees from the University of Oulu, Finland. She has been teaching at the University of Florida in Gainesville for the past 10 years. She is currently assistant professor and Director of the Cariology Program in the Department of Operative Dentistry, University of Florida, and is a Secretary for the International Association of Dental Research Cariology Group. She can be reached at jautio-gold@dental.ufl.edu.
Disclosure: Dr. Autio-Gold does not have any financial interest in products or companies listed in the article.









