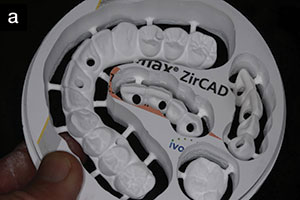
This 2-part article will discuss and present solutions for the most commonly observed techno-clinical challenges as observed by experienced team members from 4 different Authorized Lava Milling Centers (ALMCs). ALMCs are responsible for the design and milling production of copings and frameworks. The ALMCs that participated in this endeavor were (in alphabetical order) Colonial Dental Studio in Davenport, Iowa; Dental Crafters in Marshfield, Wis; Issaquah Dental Lab in Issaquah, Wash; and New Image Dental Laboratory in Atlanta.
The following question was posed as the premise for this article: “What can outsourcing dental laboratories and/or their doctors do to better assist you in providing them with the best possible milled copings and substructures?” The purpose of this question was to gain information that would be valuable in achieving better technical results, improving team relationships through mutual understanding, and optimizing patient outcomes for those receiving Lava (3M ESPE) restorations from their doctors.
Part 1 of this 2-part article will deal with challenges presented by dental laboratories that outsource the fabrication of Lava copings and frameworks to ALMCs. In some instances, where the dental laboratory contains both the milling equipment and also finalizes the restorations with fired layering porcelain, they will have likely discovered and solved some of the challenges identified. This is because the problems existed internally between departments within the same laboratory. However, depending on the laboratory, this does not necessarily mean that all the problems their prescribing doctors present, such as preparation or impression issues, have been addressed and dealt with successfully. In light of this, Part 2 (to be published in an upcoming issue of Dentistry Today) will continue with identification of, discussion of, and proposed solutions for the challenges that lie within the responsibility of doctors prescribing and preparing cases for Lava restorations. It is still prudent, however, for doctors who are using out-sourcing labs and not directly sending their work to a full-service ALMC laboratory, to be aware of the issues and solutions as presented in Part 1 of this article. This is due mainly to the team implications for the success of the finished Lava restorations.
Diagnostic skills, dental material knowledge, excellent technical and communication skills, and solid interbusiness relationships are all key ingredients needed for any team that wants to achieve optimal results with indirect restorations. With the advent of many new all-ceramic systems, such as lab-fabricated CAD/CAM restorations, we are seeing the definition and scope of our “team”widening beyond just our “local” laboratory. In the production of lab-fabricated restorations the team can now include the dental office, a “local” dental laboratory, and an additional off-site laboratory for scanning and milling various new products. For example, when Procera (Nobel Biocare) was first introduced in the 1990s to the market in North America, dies of the preparation were being scanned and computer-designed in “local” on-shore labs. The electronic data files were then transmitted to the Nobel Biocare coping production center in Sweden, where it created the lab-prescribed, densely sintered aluminum oxide crown copings. These copings (substructures) were then returned to our local labs to have the fired overlying veneer porcelain added before being returned as finished restorations to the doctors. The data files for Procera crowns and other Nobel Biocare CAD/CAM products are currently transmitted by outsourcing labs more “locally” to a production facility in New Jersey. The data and model relay loop (doctor to outsourcing lab, to milling center, back to outsourcing lab, to doctor) remains the same, despite the different production facility location and software improvements/updates in the Procera CAD/CAM process at the different “laboratory” levels.
THE LAVA SYSTEM AND OUTSOURCING LAB-ALMC RELATIONSHIPS
 |
 |
|
Figure 1. Preoperative photo (No. 30) shows an indirect composite onlay at 5 years requiring a full-coverage restoration. |
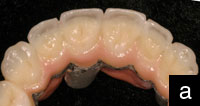
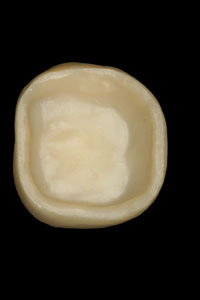
Figure 2. The completed Lava crown (view of the internal aspect). Note the more opaque appearance of the zirconium oxide coping. |
 |
|
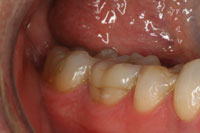
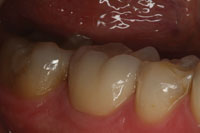
Figure 3. Postoperative photo of the completed posterior Lava crown (No. 30). |
Before beginning to discuss the identified challenges and concomitant ramifications for the ALMCs, it is appropriate and helpful to provide a brief review of the CAD/CAM Lava restorative system. We will also briefly examine the various laboratory-ALMC business models that are now actively engaged in the production of these all-ceramic restorations.
In 2000, the Lava system for crown and bridge restorations was clinically introduced in 3M ESPEís experimental operatories in Germany. The first university study began in 2001, with 5-year recall results being published in 2006.1 Five years ago in 2002, after receiving FDA approval, Lava was first introduced into the United States commercially and has become a widely used all-ceramic system. Lava is a zirconium oxide-based crown coping and bridge substructure system that provides for both underlying strength and aesthetics when veneered according to recommended techniques with the appropriate oven-fired layering porcelains. It can serve as an anterior or posterior all-ceramic alternative to porcelain-fused-to-metal crowns and bridges in many but not all circumstances (Figures 1 to 3). (Contact 3M ESPE or your dental laboratory for a list of Lava indications and contra-indications.) Lava is another example of a lab-fabricated product that goes though the same basic material-data relay loop (doctor to outsourcing lab, to milling center, back to outsourcing lab, to doctor) as described earlier.
Three different business-production models for the CAD/CAM process are available to laboratories fabricating Lava restorations:
(1) The first one is when the dental laboratory owns and houses the CAD/CAM equipment within the same full-service facility. In this model the Lava crowns and bridges are fabricated from start to finish, with the completed product delivered directly back to the doctor for delivery to the patient.
(2) Since many labs do not own their own Lava milling machines due to their physical size, financial abilities, or other reasons of choice, they find it advantageous to outsource the entire CAD/CAM process to an ALMC. Upon receipt of the necessary materials and information from the outsourcing lab, the ALMC scans, designs, and mills the Lava copings and frameworks. The completed millings can then be returned to the original outsourcing laboratory for the addition of layered porcelain and then final delivery to the doctors.
(3) The third business-production model is established when laboratories choose to buy the CAD component only (no milling machine) and become an Authorized Lava Design Center (ALDC). ALDCs are outsourcing labs performing the scanning procedure (CAD) by utilizing Scan ST (3M ESPE) equipment. Scan ST is a remote scanner using the same software as that used by the ALMCs, without the milling component. They then send the data to the ALMC to complete the actual milling process (CAM), thus saving the expense of owning milling machines. This also enables them to have direct control over the designing procedures. It serves to reduce turnaround time and allows them to keep all dental models within their lab. In addition, it gives them the ability to deal directly with any of their doctorís technical issues in a very timely manner.
(Note: 3M ESPE has been offering a choice among these 3 business-production models depending on the particular lab’s goals, needs, and abilities. It allows smaller labs to essentially ìplay on the same fieldî with the larger labs in order to provide their doctors with state-of-the-art materials.)
 |
|
Figure 4. An example of broken models that an ALMC received from a Lava outsourcing lab. ALMCs stated that this problem happens more frequently than it should. |
CHALLENGES PRESENTED TO ALMCS BY OUTSOURCING LABS (WITH SOLUTIONS)
Any problems that occur before models and/or data entered the ALMC and have not been solved at either the doctor or outsourcing lab level must ultimately be addressed by the ALMC. The outsourcing lab must then contact its doctors if and when it is required. Time and money could be saved if problems, or potential problems, can be caught at the outsourcing lab. Following are the 3 major areas that are most commonly cited by the ALMCs as needing the attention of their outsourcing labs to improve the quality and flow of work both in and out of their facilities.
Broken Models
 |
 |
|
Figure 5. Note the inability of the shipper to heed the ìFragileî marking on the side of this important package. This points out the need to employ careful packing procedures. |
Figure 6. This package shows great protection from all sides. Also note the care in packaging that has been given for all the contents. |
 |
 |
|
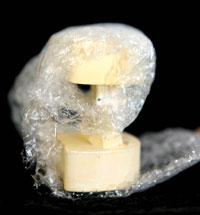
Figure 7. An example of an individual case properly packed with the Rx in a separate bag. |
Figure 8. When shipping an articulated model it is best to place protection between the teeth. |
If models for CAD are inadequately packed, they have a greater chance to arrive broken (Figure 4). Sometimes the shipping company can be less than careful about how it handles even a package marked “fragile” (Figure 5). This results in a valuable loss of production time due to the calls that have to go back and forth among all the involved parties. Unfortunately, it may often mean that the doctor will also have to be called for new impressions. This in turn can result in a loss of trust by the doctor and dental office staff that can reflect on both the outsourcing lab and the ALMC.
Solutions: The ALMCs want their outsourcing labs to ensure that all model work is properly packed for shipment. It is important to individually wrap every model and to secure the wrapping with clear tape or rubber bands. In addition, use only sturdy shipping boxes with ample protection provided by packing materials placed on all sides. (Figure 6). Do not use Fed-Ex Small Paks or other paper or fiber envelopes intended for document shipping. When sending in multiple cases in a single box, each doctor’s individual case models should be individually wrapped and put in separate bags with the prescription enclosed (Figure 7). If articulated models are shipped, place bubble wrap between the teeth as a start to wrapping the model (Figure 8).
Missing Rx Information
Believe it or not, outsourcing laboratory technicians, who themselves complain about receiving prescriptions from doctors who fail to fill them out completely, can be guilty of doing the same thing to ALMCs. Time must be allowed to pay attention to the details in this scripting procedure. Haste and omission will lead to unnecessary calls, wasting precious production time as well as negatively affecting the inter-business (outsourcing lab-ALMC-doctor) relationships.
Solutions: The outsourcing lab needs to remember why it is important for it, the ALMC, and most importantly the doctor, to fill out the ALMC prescription completely. The outsourcing lab should institute and employ a business system that requires a thorough check for completeness and accuracy before the shipping bag/box is sealed. Also, as reported by the ALMCs’ actual experiences, it is important to make certain that the Rx matches the case and model work that is being sent.
With regard to shade information on the Rx, Lava copings and frameworks can be manufactured in different shades to coordinate optimally with the layering porcelain that the outsourcing lab will be applying later. These are known as Frame Shades (3M ESPE), and are numbered from zero to 7, making a total of 8 different shade choices available. (The ìzeroî frame shade is the one most often used for overlying porcelain to be completed in the bleached shade range.) Outsourcing labs need to remember to reference a shade on the prescription to the ALMC so that millings can be shaded to coordinate aesthetically with the subsequently fired veneering porcelains.
With respect to any calls placed regarding the need for more or missing information on the prescription, common courtesy and on-time production requires that all calls should be returned promptly to the ALMC.
Improperly Done Model and Die Work
 |
 |
|
Figure 9. These dies were trimmed improperly, and the outsourcing lab or doctor outlined the margins in red prior to sending in the case (despite 3M ESPE directions to not mark the dies, which can compromise scanning). Note also that the pontic area is not sectioned and pinned. |
Figure 10. A properly trimmed die for Lava scanning. Block-out wax was used where the doctor had used a ìround-bur chamfer techniqueî and produced an undercut above the chamfer margin. |
 |
|
Figure 11. A white indicator spray has been applied to the block-out wax prior to scanning to allow for better data registration. These sprays do not seem to affect the integrity of margins. |
ALMCs stated that in some cases dies and model work (including opposing models) are not sent in with the case. Sometimes the model work is received with defects or problems of various kinds (bubbles, voids, sealer on dies) and/or the dies are not trimmed according to Lava specifications. Models for bridge work are sometimes sent into the ALMC that are not set-up correctly (pinned) for scanning (Figures 9 to 11).
Solutions: A light-colored type IV or V die stone (white, beige, buff, or green) without plastic additives (resins) should be used. Attention should be given to ensure that the model bases are smooth and flat with minimal thickness to gain maximum optical accessibility. (A common split-cast system is recommended.) For bridges the model needs to be removable from the articulator and mounting plates because the articulator will not fit in the scanner.
 |
 |
|
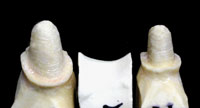
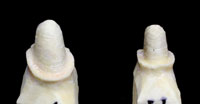
Figures 12 and 13. These photos show a properly pinned and removable pontic section involving a 3-unit Lava bridge case. This allows for complete and accurate scanning of the interproximal surfaces (including the area just below the margins) of each abutment tooth. |
All areas of the model need to be pinned and removable for scanning purposes (Figures 12 and 13). Copings or frameworks for implant abutment posts should also be pinned and removable. For large cases, the ALMC needs study models and diagnostic wax-ups to assist in properly designing the frameworks. When standard copings (only) are requested and single dies are submitted, the labial (buccal) and lingual sides should be marked accordingly to ensure orientation for proper coping design.
CONCLUSION
Excellent teamwork between the outsourcing labs and ALMCs is important to reduce mutual stress and to maximize profits. To serve their doctors better, and to assist ALMCs in providing the quality service that they are being expected to complete in a timely fashion, outsourcing laboratories should perform preshipment checks (QCs) on every case. Inspection and evaluation for completeness, quality, accuracy, and proper shipping preparation of all materials being sent into the ALMC is time well spent.
Reference
- Nothdurft FP, Rountree PR, Pospiech PR. Clinical long-term behavior of Zirconia-based bridges (LAVA): five years results. Presented at: IADR Pan European Federation; September 14, 2006; Dublin, Ireland. Abstract 0312. http://iadr.confex.com/iadr/pef06/techprogram/abstract_85451.htm. Accessed August 17, 2007.
Additional Reading
2. ESPE Dental Products. Lava All-Ceramic System Laboratory Preparation [Technical Guidelines No. 70-2009-3507-3]. St Paul, Minn; 3M: 2002. Published. PDF: http://multimedia.mmm.com/mws/mediawebserver.dyn?6666660Zjcf6lVs6EVs666JM9COrrrrQ-.
Acknowledgment
The author wishes to thank all those experienced personnel from the Authorized Lava Milling Centers who participated in this project by providing candid information, technical advice, and photos.
Dr. Adams, a graduate of the University of Michigan, is an assistant clinical professor in the Dental Residency Program at the University of Toledo College of Medicine. He lectures internationally emphasizing doctor-technician relationships and techno-clinical perspectives. He also facilitates hands-on preparation workshops designed to optimize the utilization of all-ceramic systems. In addition to his years in private practice, he has had the opportunity to serve as a doctor-technician liaison for many dental laboratories throughout North America for more than 12 years. Dr. Adams is a Contributing Editor for Dentistry Today and is listed in Dentistry Today’s Top Clinicians in Continuing Education (2004 to 2007). In addition, he also serves on the Advisory Board for Spectrum Dialogue (Palmeri Publications). Dr. Adams is a member of the ADA, AGD, AACD, ICOI, ICD, and the National Association of Dental Laboratories.
Disclosure: Dr. Adams owns no financial interest in the companies he refers to in this article.