INTRODUCTION
In the early 2000s, the field of dentistry was shocked when published reports of severe complications and morbidity surfaced with patients on a newer therapeutic antiresorptive medication (bisphosphonates). Marx1 and Ruggiero et al2 were the first to report patients taking bisphosphonates for cancer treatment (IV) or osteoporosis (oral) becoming susceptible to bone necrosis lesions termed medication-related osteonecrosis of the jaw (MRONJ). Since that time, there has been significant debate and controversy with no general consensus on how bisphosphonate patients should be treated (eg, no treatment, no drug modification, drug holiday, C-Telopeptide [CTX] Test) for dental procedures in implant dentistry.
Currently, the field of implant dentistry is entering a new challenge, which could be far more significant and controversial than bisphosphonates. A relatively newer class of therapeutic drugs (“biologics”) has been introduced in the medical world for the treatment of a wide spectrum of diseases, including autoimmune, inflammatory, dermatologic, and infectious diseases and neoplasms. These novel medications utilize living organisms and recombinant DNA in their manufacturing processes. Unlike conventional medications that target the entire immune system (eg, steroids, methotrexate, and cyclosporine), biological therapeutics are significantly more beneficial as they only target particular portions of the immune system. This most commonly will result in fewer side effects for the patient.3
Biological therapeutics are the fastest-growing sector in the pharmaceutical industry, with total revenues exceeding $163 billion annually. The annual growth rate is staggering at 8%, far greater than conventional pharmaceuticals, which approximate 4%. Since 1995, the applications for biologic therapeutic patents have increased by more than 25% per year. There are currently more than 200 approved biologic drugs on the market, with approximately 1,500 drugs being evaluated in clinical trials and many more drugs in the pipeline.4 The ability of these drugs to treat previously untreatable diseases is what is fueling this growth. With the unlimited scope and potential of these medications, more targeted, effective, and personalized biologic medications will be developed in the future to treat even the most complex diseases.5
BIOLOGIC MEDICATIONS
Biologic medications have been approved for a wide array of conditions and disease processes. Biologics are most commonly classified by their suffixes as to their origin: human (“mab”), humanized (“zumab”), or chimeric (“ximab”). Table 1 describes the most common biologics and their intended targeted conditions and diseases.
Possible Side Effect Complications in Dentistry
Although biologic drugs have far fewer side effects than conventional pharmaceuticals, they do specifically have potential adverse effects on normal bone physiology. There exist many case reports in the medical literature concerning postoperative infections involving biologic medications, especially following orthopedic surgery. Giles et al6 showed a significant increase in post-op infections after orthopedic surgery procedures. Ruyssen-Witrand et al7 also reported an increased infection rate in rheumatoid arthritis patients undergoing orthopedic joint surgery.
In the dental literature, most documentation is via case reports, as little research or data exists on how biologic medications may affect patients undergoing invasive dental procedures (eg, extractions, dental implants, and bone grafts). Ciantar and Adlam8 reported a case of mandibular osteomyelitis on a patient being treated with Infliximab for juvenile arthritis. Sri et al9 reported one of the first cases involving an extraction and the development of a mandibular osteomyelitis in a patient being treated for psoriasis with biologic medications (Figure 1).
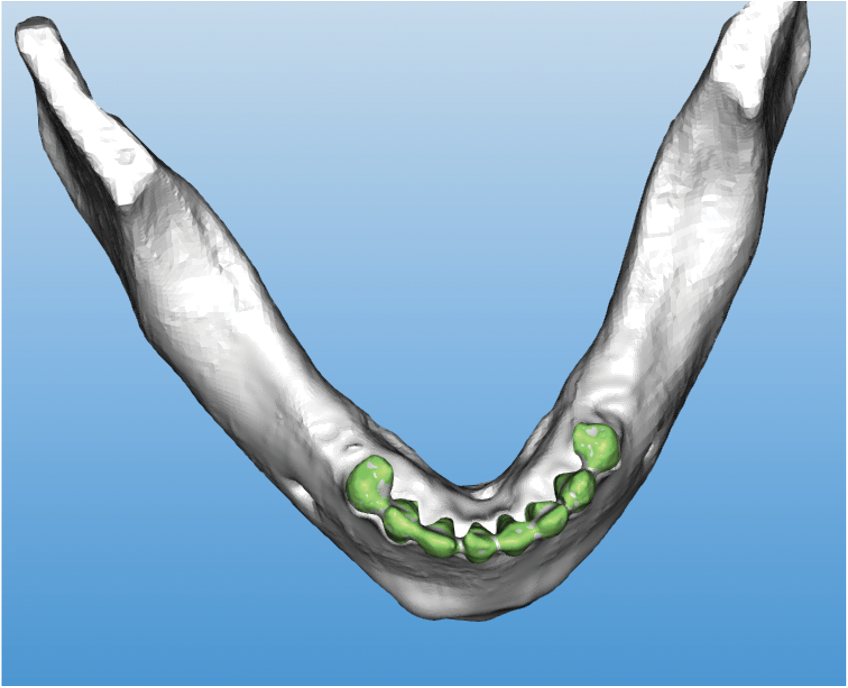
Figure 1. (a) Preoperative image prior to extraction of mandibular teeth.
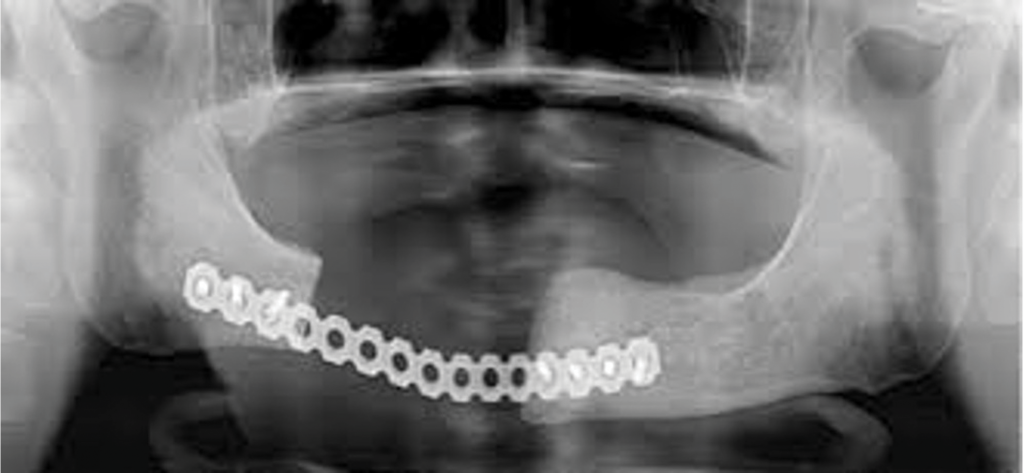
Figure 1. (b) Post-extraction osteomyelitis resulting from the patient being treated with Etanercept.
Tsuchiya et al10 related a case of suppurative osteomyelitis derived from periapical periodontitis in a patient being treated for Crohn’s disease. Ziobrowska-Bech et al11 presented a 10-year study showing a relationship between biologic drugs and children with chronic noninfectious osteomyelitis with either mono- or multifocal bone lesions.
Cillo and Barbosa12 reported on the first documented implant-related complication with a biologic medication. An adalimumab-related dental implant surgical-site infection presented in a patient 2 weeks after extractions and immediate placement of 5 mandibular implants (Figure 2).
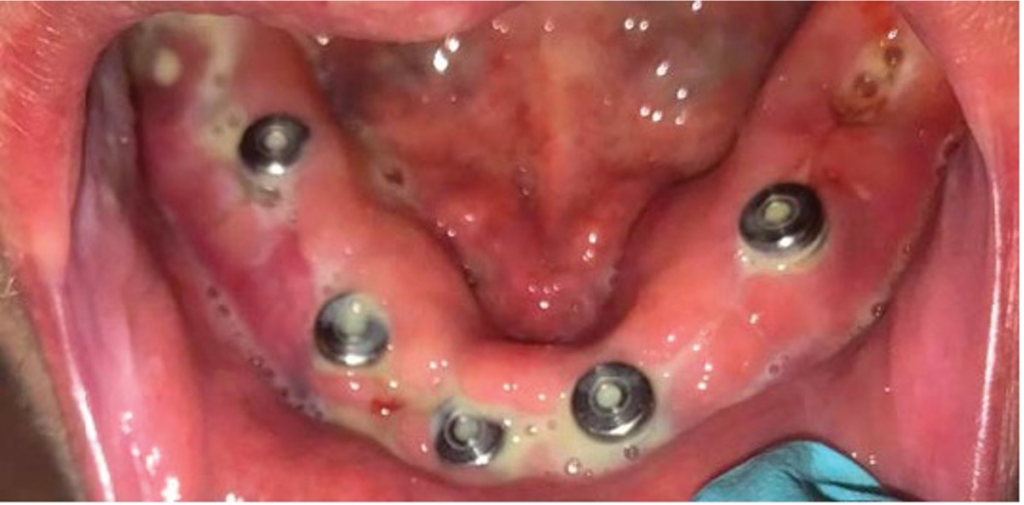
Figure 2. (a) Patient presented with draining mandibular exudate to Allegheny General Hospital (Pittsburgh) emergency department 2 weeks after extraction and placement of 5 mandibular implants.
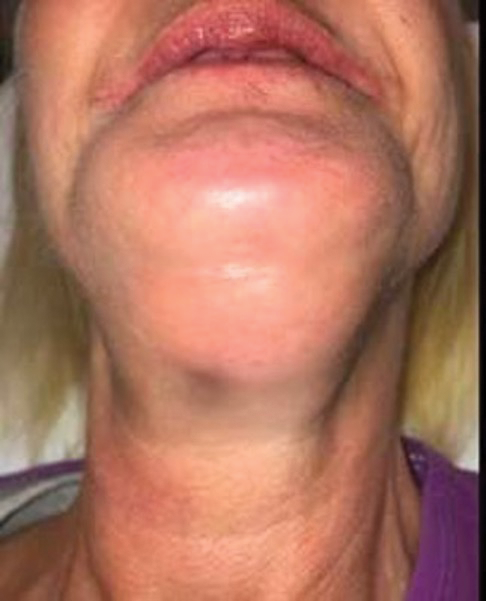
Figure 2. (b)
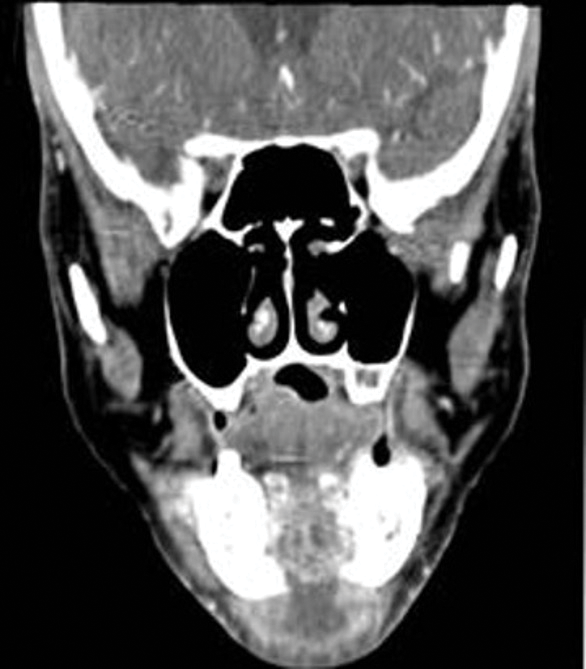
Figure 2. (b and c) Extraoral image and CT scan depicting significant fluid collection inferior and posterior to the mandibular symphysis with extension of submental abscess into the bilateral submandibular spaces.
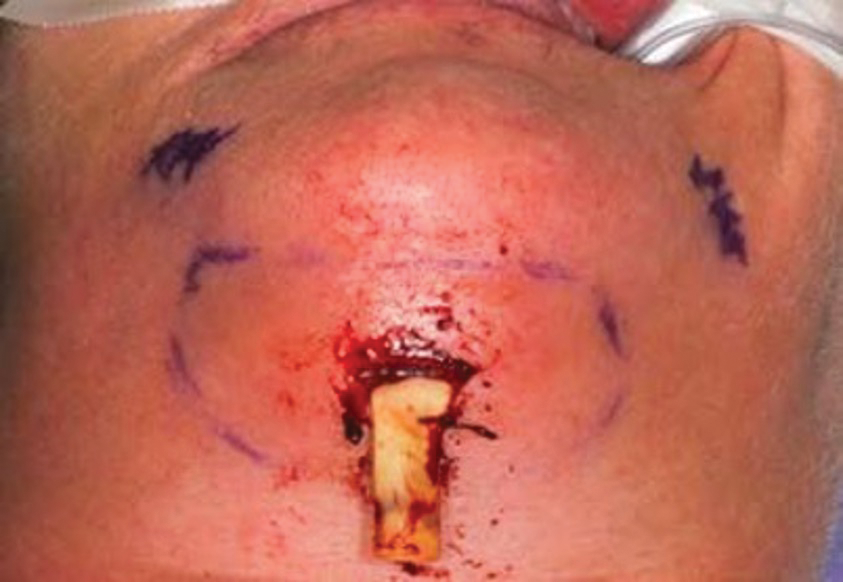
Figure 2. (d) Extraoral drainage with implant removal was completed. (Courtesy of Joseph E. Cillo Jr., DMD, MPH, PhD.)
After extraoral incision and drainage of the involved fascial spaces, implant explantation and debridement of necrotic mandibular bone were completed. The patient healed uneventfully after treatment. Therefore, it appears a definite association exists between biologic medications and post-op infections after invasive dental surgery; however, without controlled, prospective cohort studies, a true prevalence and relationship cannot be established.
POSSIBLE PHARMACOLOGIC RELATIONSHIP
So how does this newest classification of medications result in post-op dental complications? Specifically, biologic medications are tumor necrosis factor (TNF) inhibiting drugs that utilize the immune system’s natural processes to detect and destroy abnormal cells.13 TNF plays a central role in the body’s ability to fight infection. When this factor is inhibited (as with biologic medications), an increased susceptibility to infection may result. Multiple studies have shown a direct association between TNF inhibitors and interference with normal bone physiology. Biologic medications have been reported in the literature to decrease bone turnover,14-16 have direct effects on the viability of osteocytes,17 and inhibit osteoclastgenesis.18 Therefore, these detrimental effects on the bone-healing process closely parallel the genesis of MRONJ with antiresorptive (bisphosphonate) medications.
PREOPERATIVE PREVENTIVE MEASURES FOR BIOLOGIC MEDICATION PATIENTS
With the number of dental implant procedures increasing significantly every year, clinicians must be aware of the possible sequelae of patients taking biologic medications and undergoing invasive dental procedures. Due to the increased risk of post-op infection, clinicians must work closely with the patient’s physician in the development of a treatment plan that is case-specific for the reduction of post-treatment complications. Medical consultation and clearance are highly recommended prior to any proposed implant treatment, especially on patients taking biologic medications.18
If a patient presents with a past history of biologic medication use but is not currently being treated with the medications, medical consultation is still recommended to ascertain the level of immune suppression and current status of the patient’s disease process. A detailed verbal and written informed consent is suggested.
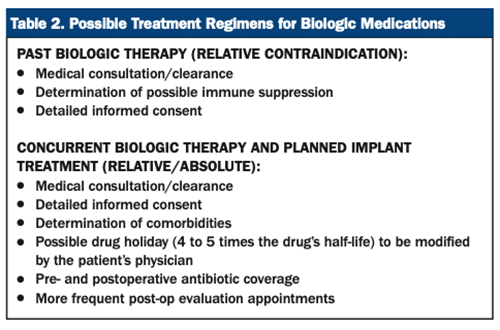
For patients currently taking biologic medications and who have future invasive surgical procedures planned, medical consultation/clearance is highly recommended. There exist many factors the physician will take into consideration in the determination of the best course of treatment for the patient. In general, this should include the specific medication in question (ie, medication toxicity and strength and the potential for adverse effects), medication dosage, treatment duration, comorbidities, the possibility of disease rebound, and type of surgical procedure to be performed. In most cases, modification of the medication is indicated in the perioperative period, which includes a discontinuation via a drug holiday. Smith et al19 recommends a drug discontinuation of approximately 4 to 5 times the half-life of the medication.
For example, possible discontinuation (drug holiday) prior to surgery for the most common biologics would include etanercept (2 weeks), adalimumab (6 to 8 weeks), and infliximab (4 to 6 weeks).20 The patient’s physician should instruct the patient on the specific instructions of the drug holiday. In no situation should a dental provider modify a patient’s biologic medication that the patient’s physician prescribes. In addition, detailed verbal and written informed consent should be presented to the patient. Biologic therapy is most commonly restarted post-op when satisfactory wound healing is present with no evidence of infection (see Table 2).
CONCLUSION
In conclusion, biologic drugs are an exciting new class of medications that have revolutionized the treatment of a wide spectrum of conditions and diseases in medicine. In the future, more and more of our dental patients will be utilizing these medications for a full array of disease processes as this promising classification of drugs will continue to grow and become more complex. Therefore, dentists must be aware of the possible complications that may arise from these medications, especially in combination with dental implant procedures.
Unfortunately, there exists minimal research that can quantify the relationship extent between post-op infections and the use of biologic medications. Caution must be used when treatment planning extractions, dental implants, and bone grafting procedures with concomitant use of biologic medications. Post-op infection is the most common adverse effect; therefore, clinicians must be prudent in evaluating the patient pre- and post-op along with consultation with the patient’s physician. In the future, a clearer picture will be determined of the association and risk between TNF-alpha inhibitor therapy and postsurgical infections as more research is completed on this topic.
REFERENCES
1. Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–7. doi:10.1016/s0278-2391(03)00720-1
2. Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527–34. doi:10.1016/j.joms.2004.02.004
3. Craik DJ, Fairlie DP, Liras S, et al. The future of peptide-based drugs. Chem Biol Drug Des. 2013;81(1):136–47. doi:10.1111/cbdd.12055
4. Otto R, Santagonisto A, Schrader U. Rapid growth in biopharma: Challenges and opportunities. In: From Science to Operations: Questions, Choices and Strategies for Success in Biopharma. McKinsey Co.; 2014.
5. Kesik-Brodacka M. Progress in biopharmaceutical development. Biotechnol Appl Biochem. 2018;65(3):306–22. doi:10.1002/bab.1617
6. Giles JT, Bartlett SJ, Gelber AC, et al. Tumor necrosis factor inhibitor therapy and risk of serious postoperative orthopedic infection in rheumatoid arthritis. Arthritis Rheum. 2006;55(2):333–7. doi:10.1002/art.21841
7. Ruyssen-Witrand A, Gossec L, Salliot C, et al. Complication rates of 127 surgical procedures performed in rheumatic patients receiving tumor necrosis factor alpha blockers. Clin Exp Rheumatol. 2007;25(3):430–6.
8. Ciantar M, Adlam DM. Treatment with infliximab: Implications in oral surgery? A case report. Br J Oral Maxillofac Surg. 2007;45(6):507–10. doi:10.1016/j.bjoms.2006.06.004
9. Sri JC, Tsai CL, Deng A, et al. Osteomyelitis occurring during infliximab treatment of severe psoriasis. J Drugs Dermatol. 2007;6(2):207–10.
10. Tsuchiya S, Sugimoto K, Omori M, et al. Mandibular osteomyelitis implicated in infliximab and periapical periodontitis: A case report. J Oral Maxillofac Surg Med Pathol. 2016; 28(5):410–5. doi:10.1016/j.ajoms.2016.03.003
11. Ziobrowska-Bech A, Fiirgaard B, Heuck C, et al. Ten-year review of Danish children with chronic non-bacterial osteitis. Clin Exp Rheumatol. 2013;31(6):974–9.
12. Cillo JE Jr, Barbosa N. Adalimumab-Related Dental Implant Infection. J Oral Maxillofac Surg. 2019;77(6):1165–9. doi:10.1016/j.joms.2019.01.033
13. Mazurek J, Jahnz-Różyk K. The variety of types of adverse side-effects during treatment with biological drugs. Int Rev Allergol Clin Immunol Family Med. 2012;18:35–40.
14. Luo G, Li F, Li X, et al. TNF‑α and RANKL promote osteoclastogenesis by upregulating RANK via the NF‑κB pathway. Mol Med Rep. 2018;17(5):6605–11. doi:10.3892/mmr.2018.8698
15. Hehlgans T, Pfeffer K. The intriguing biology of the tumour necrosis factor/tumour necrosis factor receptor superfamily: players, rules and the games. Immunology. 2005;115(1):1-20. doi:10.1111/j.1365-2567.2005.02143.x
16. Liao HJ, Tsai HF, Wu CS, et al. TRAIL inhibits RANK signaling and suppresses osteoclast activation via inhibiting lipid raft assembly and TRAF6 recruitment. Cell Death Dis. 2019;10:77. doi:10.1038/s41419-019-1353-3
17. Marahleh A, Kitaura H, Ohori F, et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front Immunol. 2019;10:2925. doi:10.3389/fimmu.2019.02925
18. Ohori F, Kitaura H, Ogawa S, et al. IL-33 inhibits TNF-α-induced osteoclastogenesis and bone resorption. Int J Mol Sci. 2020;21:1130. doi:10.3390/ijms21031130
19. Smith CH, Anstey AV, Barker JN, et al. British Association of Dermatologists’ guidelines for biologic interventions for psoriasis 2009. Br J Dermatol. 2009;161(5):987-1019. doi:10.1111/j.1365-2133.2009.09505.x
20. Radfar L, Ahmadabadi RE, Masood F, et al. Biological therapy and dentistry: a review paper. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(5):594-601. doi:10.1016/j.oooo.2015.07.032
ABOUT THE AUTHOR
Dr. Resnik received his dental degree from the University of Pittsburgh School of Dental Medicine. Upon graduation from dental school, he continued his training at the university, receiving a specialty degree in Prosthodontics, a surgical fellowship in Oral Implantology, and a Master’s degree in Oral Implantology/Radiology. He has been faculty and surgical director of the Misch Resnik Implant Institute for more than 30 years. He is also director of the Resnik Hands-On Surgical Implant Boot Camps in Ohio and the Dominican Republic.
Dr. Resnik maintains faculty positions at numerous universities, including the University of Pittsburgh (Graduate Prosthodontics), Temple University (Oral Implantology and Periodontics), and Allegheny General Hospital (Oral & Maxillofacial Surgery) in Pittsburgh.
Along with his passion for lecturing and education, Dr. Resnik is also an accomplished author, having published more than 200 articles, and his recent textbooks, Avoiding Complications in Oral Implantology and 4th Edition of Contemporary Implant Dentistry, are best sellers in the field of implant dentistry.
Dr. Resnik currently maintains a private practice in Orlando. He can be reached at resnikdmd@gmail.com.
Disclosure: Dr. Resnik reports no disclosures.












