INTRODUCTION
Complete edentulism influences the patient’s physical and psychological health. Rehabilitation through removable prostheses can have many associated disadvantages, such as a loss of the taste of food, a decrease in the effectiveness of chewing, mobility of the prostheses, alterations in phonation (dyslalia), resorption of the alveolar process, and the possible appearance of ulcers in the oral mucosa due to rubbing of the prosthesis,1 as well as other factors of a psychological nature. To avoid such complications, there are various alternative treatments with implants, such as overdentures, fixed prostheses, or hybrid prostheses. Concerning the latter, currently, with the development of digital systems, there has been a great advance in biomaterials and an increasing tendency to avoid the use of metal structures due to a lower aesthetic2,3; a greater weight2-4; mechanical, thermal, biological, and electrical properties2,3; a greater allergenic potential (specifically, type IV allergies)2-4; and a longer processing time in the laboratory. In addition, metals are harder and insulate worse from temperature than natural teeth.4 Various alternatives to using metallic materials have been proposed, such as the use of ceramics or resins. In this area of alternatives, a nanohybrid biopolymer reinforced with several layers of multidirectional glass fibers bonded by epoxy resin and adapted to modern CAD/CAM systems has been developed.2,3 Due to its flexural strength, it is a biomaterial with characteristics comparable to dentin, and its function could be compared to that of Sharpey’s fibres.4
This article aims to show the treatment of a patient with lamellar implant failure in the second sextant, which was replaced by short implants and a hybrid implant prosthesis of biopolymer reinforced with fiberglass (BRWF).
CASE REPORT
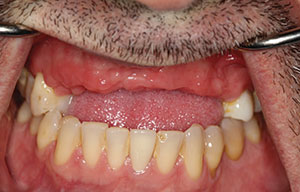
The patient was a 71-year-old woman with no known allergies and no clinically relevant operations or harmful habits. She presented with arterial hypertension (being treated with an Angiotensin II receptor inhibitor: Parapres 16 mg) and hypercholesterolemia (Simvastatin 20 mg) and was being treated with a vitamin D supplement (Hydroferol 0.26 mg). Her teeth Nos. 4, 5, and 13 were extracted due to infectious pathology. She was looking for solutions regarding aesthetic and functional problems as she was carrying some laminar implants in the second sextant that have failed after 30 years in the mouth (Figure 1).
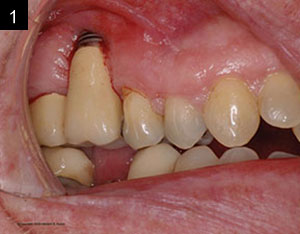
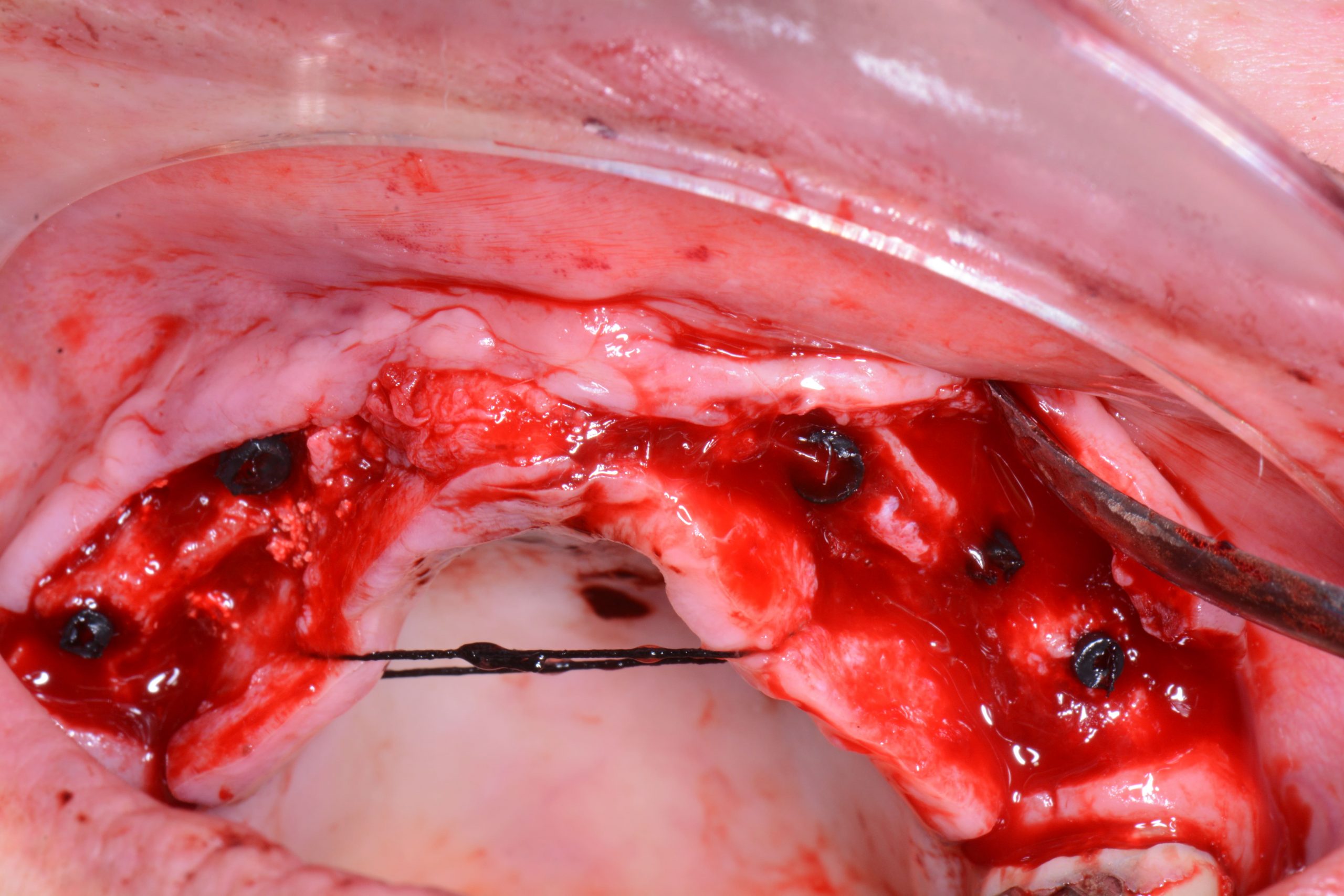
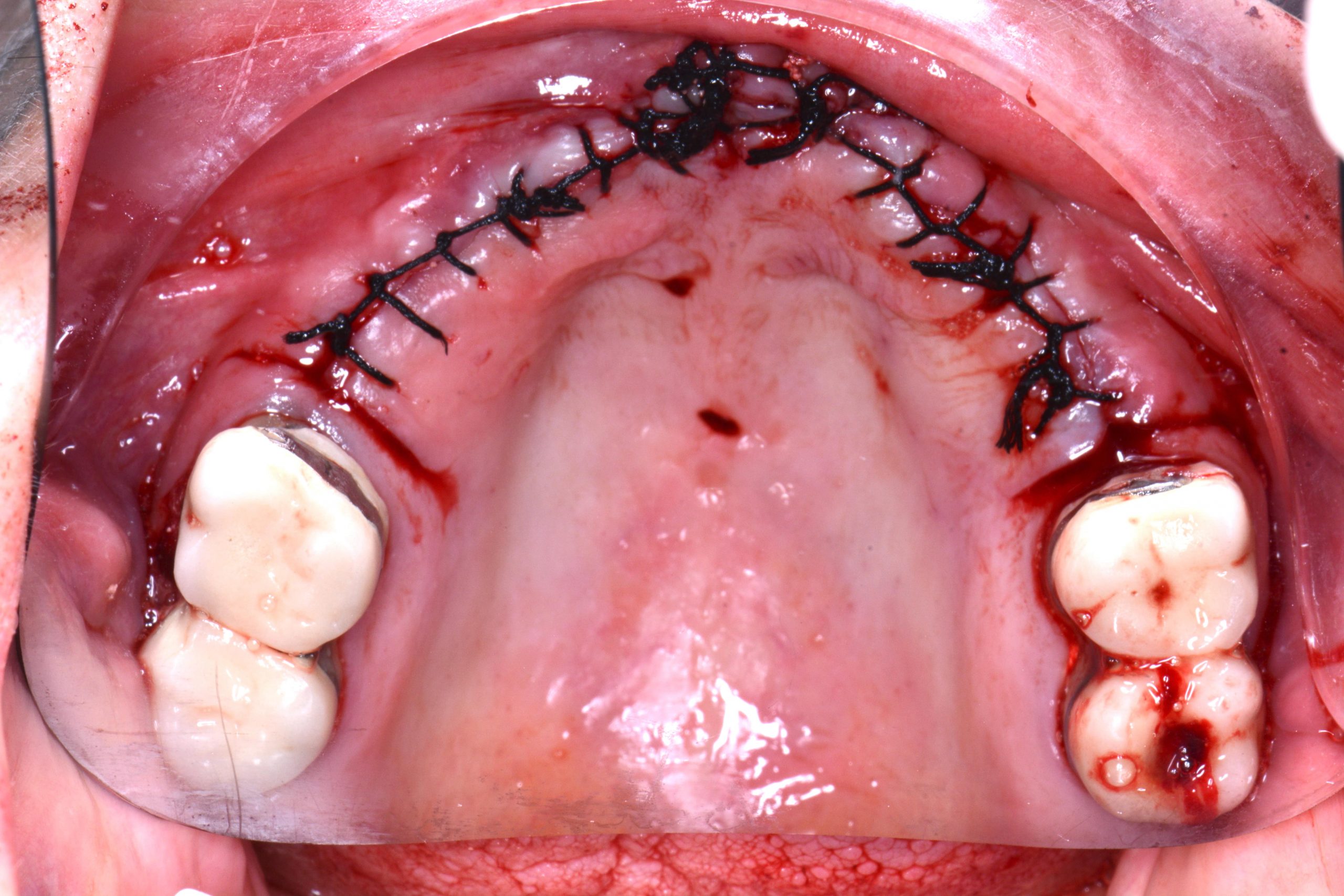
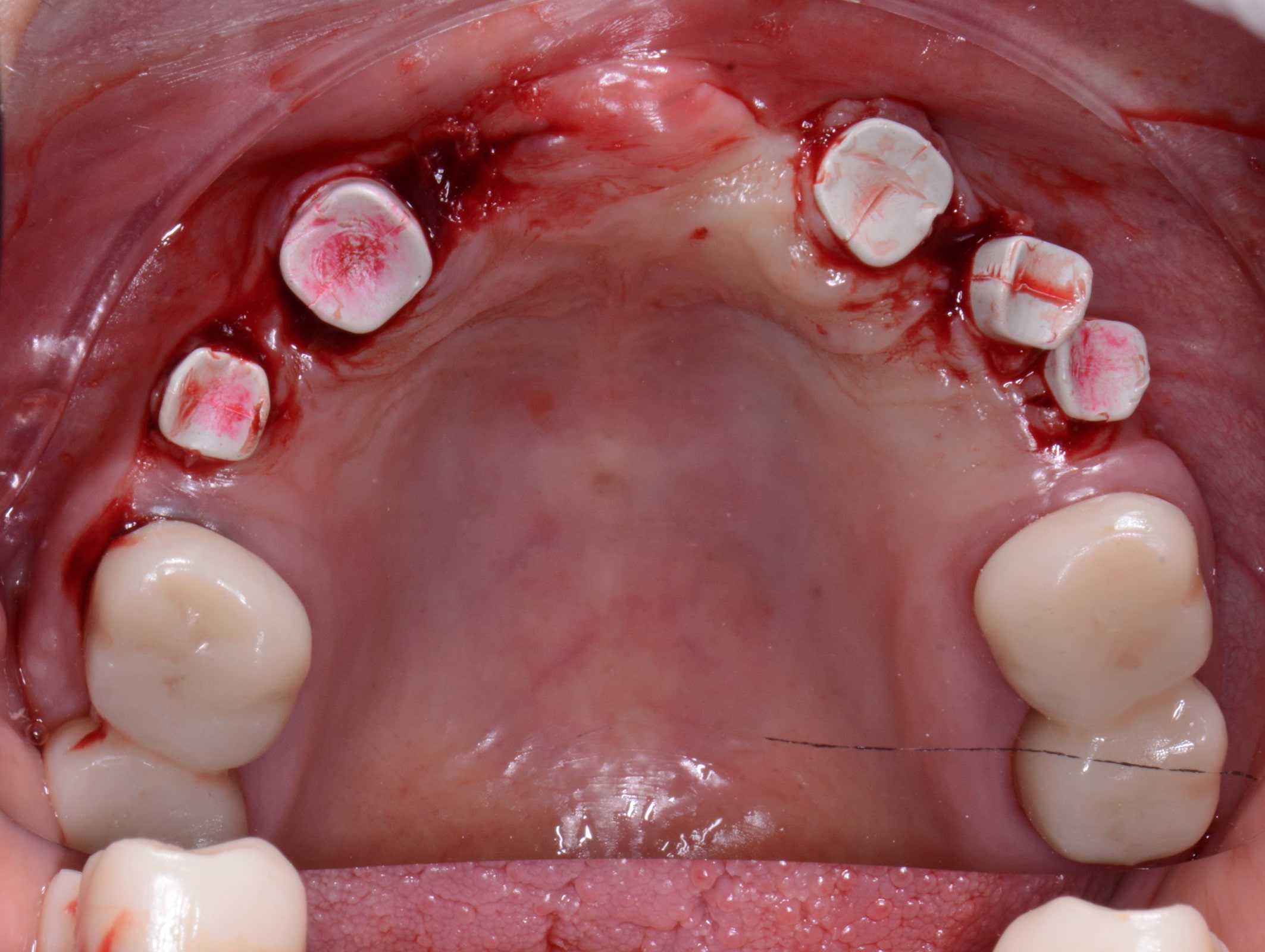
After a complete anamnesis, a physical examination, study models, and radiological tests5 (Figure 2), the following treatment plan (steps a to i) was carried out in the upper arch: (a) periodontal treatment; (b) multiple root canal treatment of teeth Nos. 2, 3, and 14; (c) root canal retreatment of tooth No. 15; (d) exodontia of the laminar implants of the second sextant (Figure 3) and placement of a removable mucosa-borne partial acrylic prosthesis to restore aesthetics and function immediately; and (e) after 5 months, planning the implant treatment. The CBCT scan showed a type IV to V alveolar ridge morphology, according to Atwood’s6 classification (Figures 4 and 5). Antibiotic prophylaxis (amoxicillin 3g one hour before surgery7) and rinsing with 0.12% chlorhexidine, in combination with 0.05% cetylpyridinium chloride, twice a day for 15 days, starting the day before surgery, were prescribed preoperatively. A mid-crestal ridge incision was made from the mesial area of 3 to 14 with papilla preservation to elevate a mucoperiosteal flap and facilitate the exposure of the alveolar ridge. Five short Bicon Dental Implants were inserted (Figure 6) in the areas corresponding to #4i (3.5 × 8 mm, located 2 mm subcrestally), #6i (5 × 6 mm, 3 mm subcrestal), #10i (4.5 × 6 mm, 2 mm subcrestal), #11i (3.5 × 8 mm, 1 mm subcrestal), and #13i (3.5 × 8 mm, 2 mm subcrestal). The #6i and #13i presented bone dehiscences on the vestibular cortex at the time of insertion, so guided bone regeneration was performed at this level with ß-tricalcium phosphate bone chips (SynthoGraft [Bicon Dental Implants]) and using a collagen membrane (Creos [Nobel Biocare]) as a barrier. The flap was repositioned and sutured with continuous 4-0 silk blocking stitches (Figure 7). Continuing with the treatment plan steps, (f) the temporary removable prosthesis was relieved to avoid pressure areas on the implants, and it was relined with a temporary soft denture liner (Figure 8). During the healing period, the fixed dental prostheses in both maxillary posterior sectors were replaced with separate and individual lithium disilicate crowns (IPS e.max CAD LT A2 [Ivoclar Vivadent]). (g) After 5 months of surgery, the second phase of the implants was carried out, and the healing caps were placed (Figure 9). A vestibuloplasty was performed without placing a free gingival graft in the second sextant in order to increase the width of the inserted mucosa and improve the long-term maintenance of the implants (Figure 10). The provisional prosthesis was relined again with Visco-Gel to adapt it to the new situation. (h) After 15 days, conventional silicone impressions were taken to start the prosthetic phase. Plastic impression abutments with a “basket” shape designed to be captured by the impression material were employed. Even though there were several implants, these must not be splinted. (i) After various tests, a hybrid implant prosthesis type PF-3 (according to the Misch8 classification) from BRWF (Trinia [Dental Tonal]) was placed.
 |
 |
| Figure 1. Initial intraoral situation. | Figure 2. Initial orthopantomography (OPG). |
 |
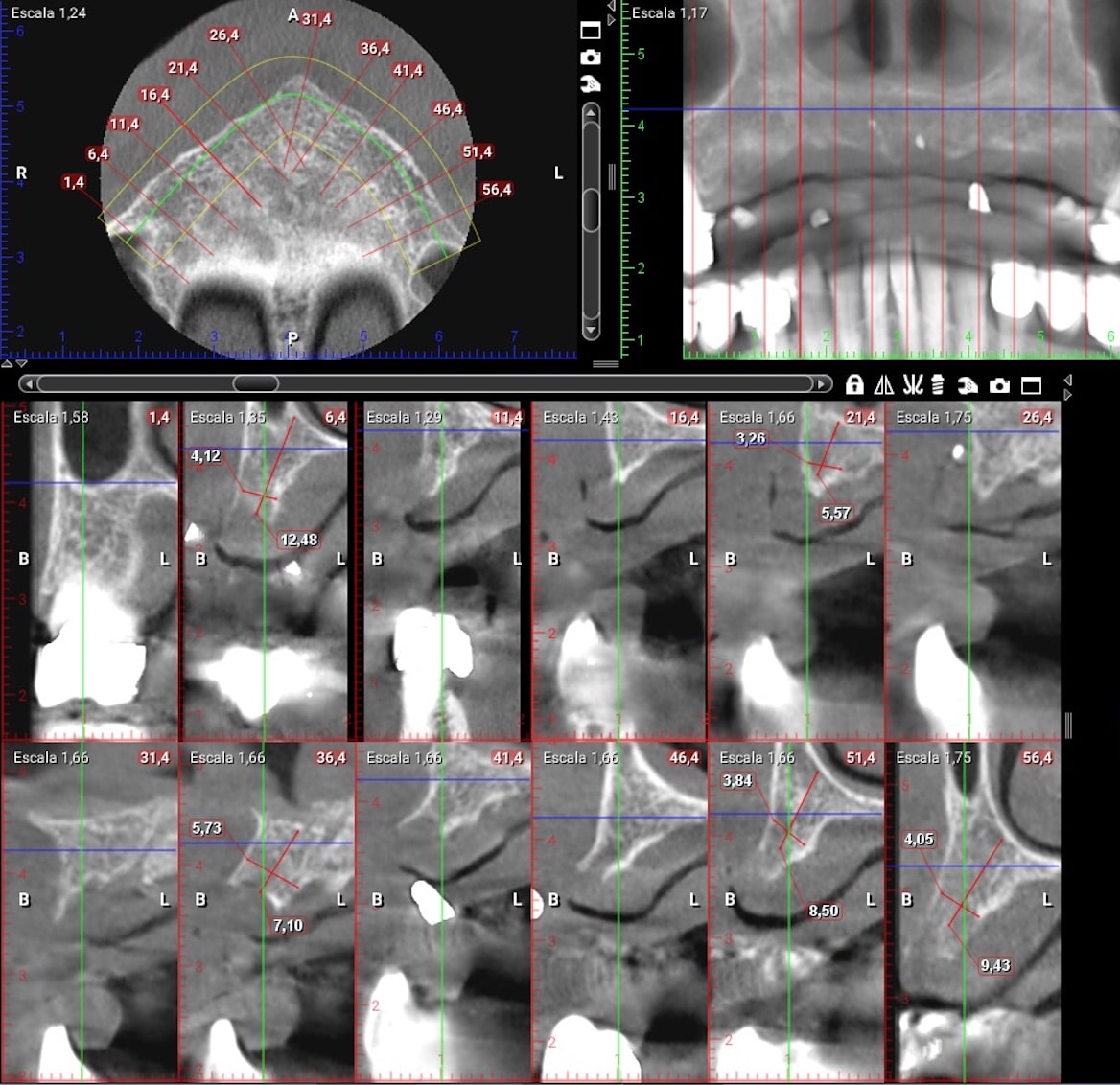 |
| Figure 3. Failed laminar implants. | Figure 4. CBCT scan (Romexis [Planmeca]) showing measurements in the implant sites after 5 months. |
 |
 |
| Figure 5. Clinical situation 5 months after the explantation of the laminar implants. | Figure 6. Bicon implants, inserted with plastic closure caps in place. |
 |
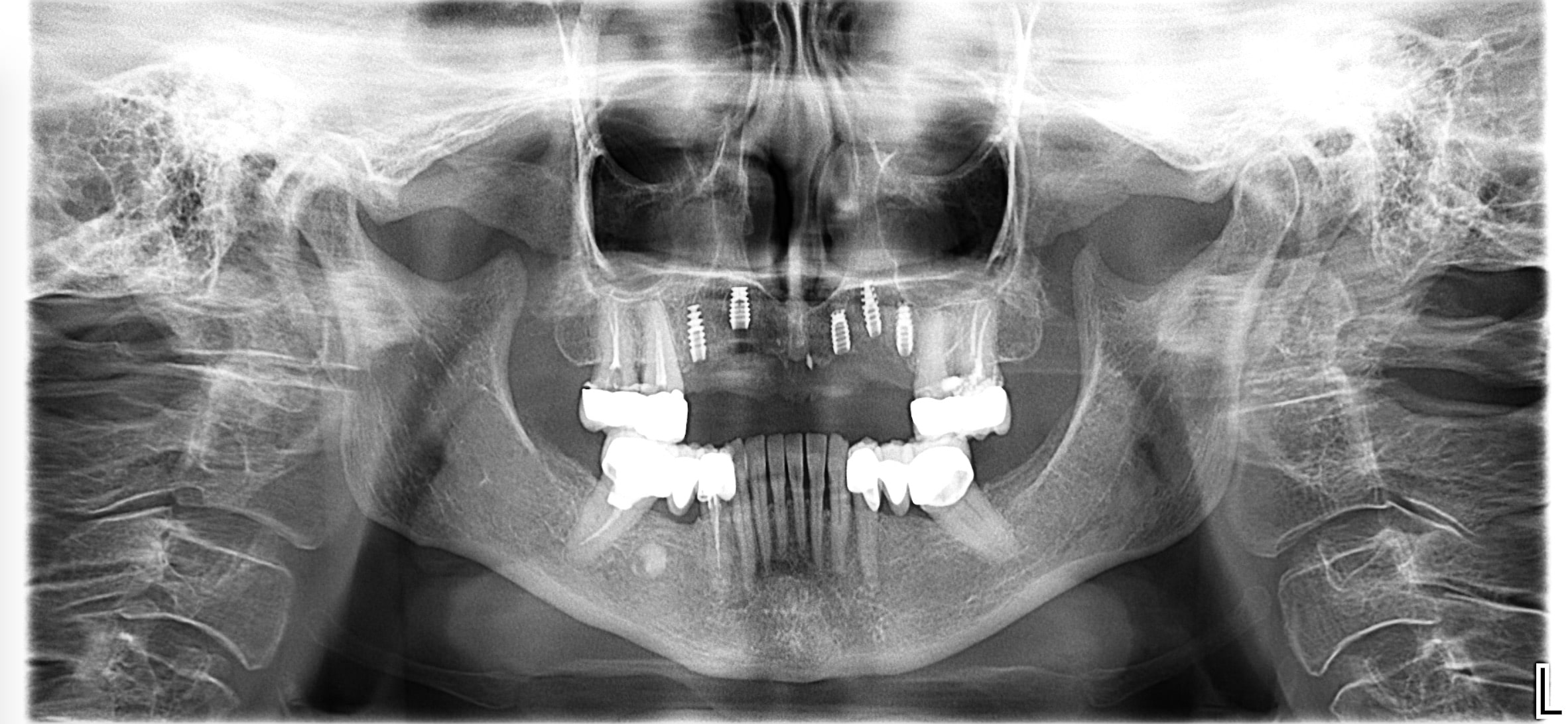 |
| Figure 7. Immediate postoperative period. | Figure 8. OPG performed immediately post-op. |
 |
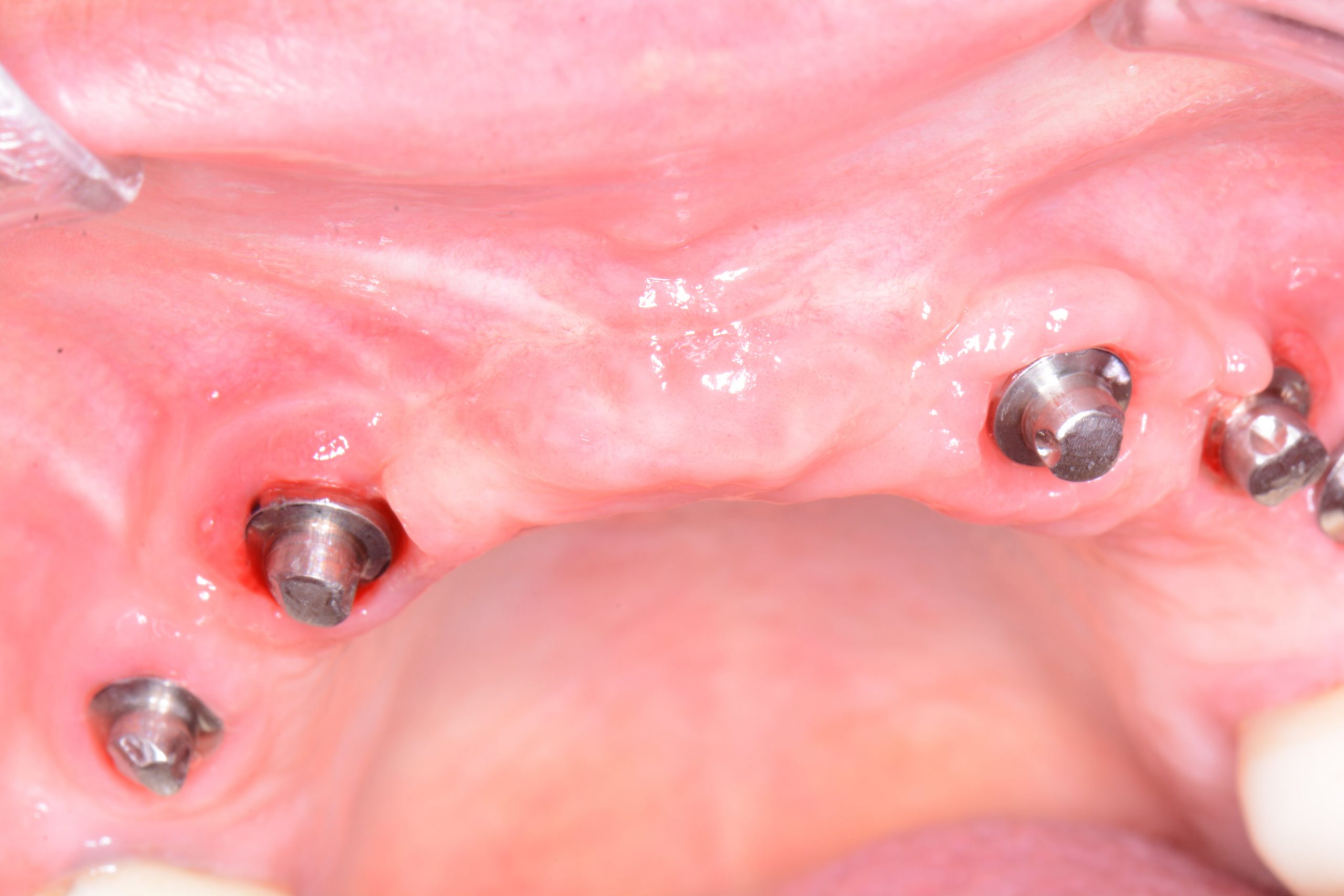 |
| Figure 9. Placement of the healing caps on the implants 5 months after their insertion. | Figure 10. Occlusal view 15 days after implant exposition and vestibuloplasty of the second sextant. |
Table 1. Comparison of mechanical properties between biopolymer reinforced with fiberglass (BRWF), alveolar bone, and different biomaterials commonly used as implant substructures (cobalt-chrome [CoCr], polyether ether ketone [PEEK], megapascals [MPa], gigapascals [GPa], not specified [N/S], Vickers hardness—the resistance of a material to be penetrated as measured in Vickers units of hardness [VHN], and Poisson coefficient the relation between lateral and axial deformation in a specimen with axial load.
|
Properties |
BRWF |
Bone Tissue |
Zirconium oxide |
CoCr alloy |
TriLor |
PEEK ivory |
|
Bending Force (MPa) |
393 MPa |
170 MPa |
750 to 1,465 MPa |
500 to 800 MPa |
540 MPa |
200 MPa |
|
Young’s Modulus (GPa) |
18.80 GPa |
14.80 to 20 GPa |
200 GPa |
210 GPa |
26 GPa |
5 GPa |
|
Tensile Strength (MPa) |
169 MPa |
90 to 190 MPa |
115 to 71 MPa 1 |
1,100 to 1,900 MPa |
380 MPa |
115 MPa |
|
Density (g/cm3) |
1.68 g/cm3 |
1.70 g/cm3 |
5 to 6.15 g/cm3 |
10 g/cm3 |
N/S |
N/S |
|
Fracture Resistance (MPa•m1/2) |
9.70 MPa•m1/2 |
6.40 MPa•m1/2 |
6 to 10 MPa•m1/2 |
6 MPa•m1/2 |
N/S |
N/S |
|
Vickers hardness (VHN) |
347 VHN |
120 to 160 VHN |
12 VHN |
420 VHN |
N/S |
N/S |
|
Compression resistance (MPa) |
347 MPa |
170 MPa |
1,200 to 5,200 MPa |
7,000 MPa |
530 MPa |
256 MPa |
|
Poisson coefficient |
N/S |
0.10 to 0.20 |
0.22 to 0.32 |
0.27 to 0.30 |
N/S |
N/S |
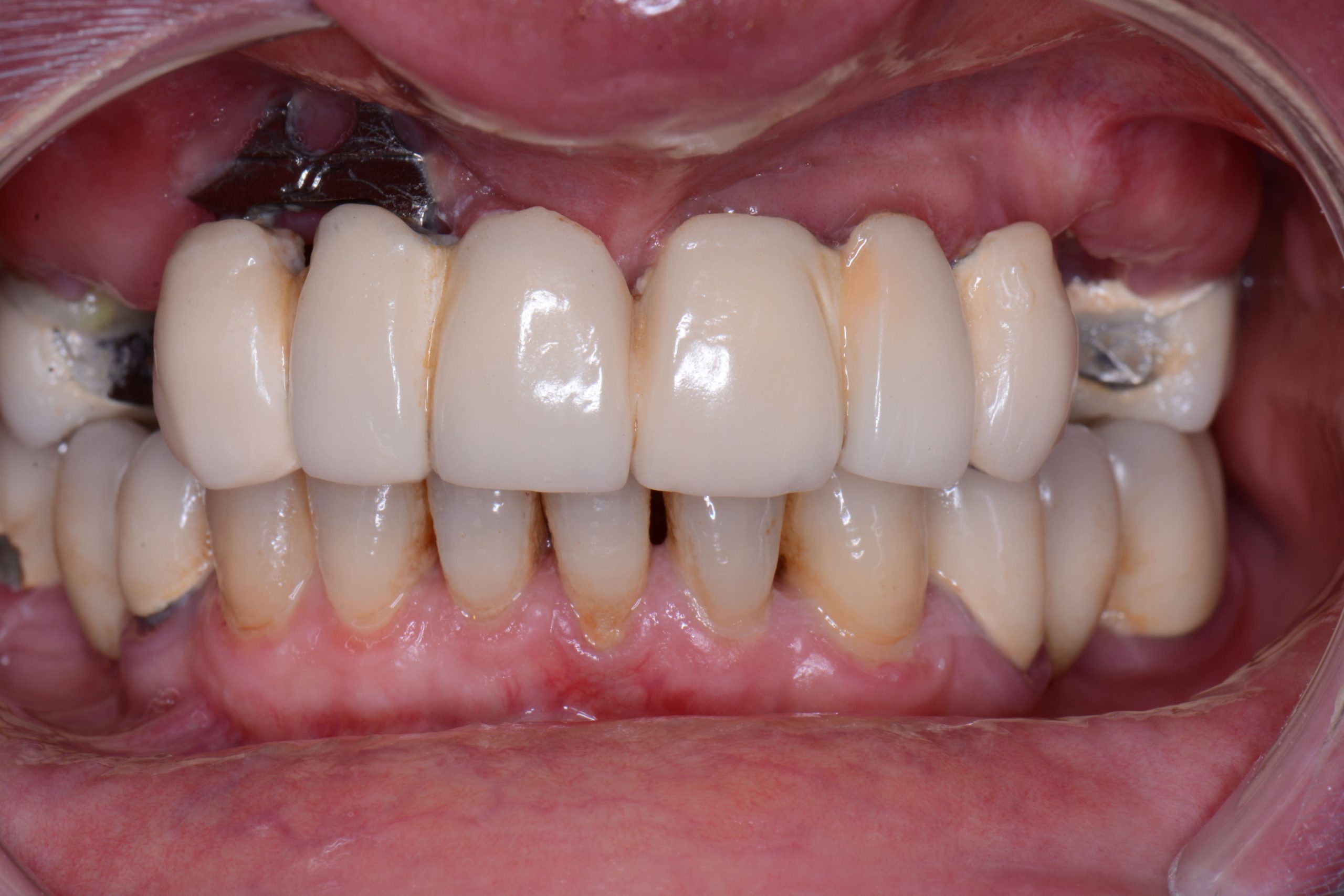
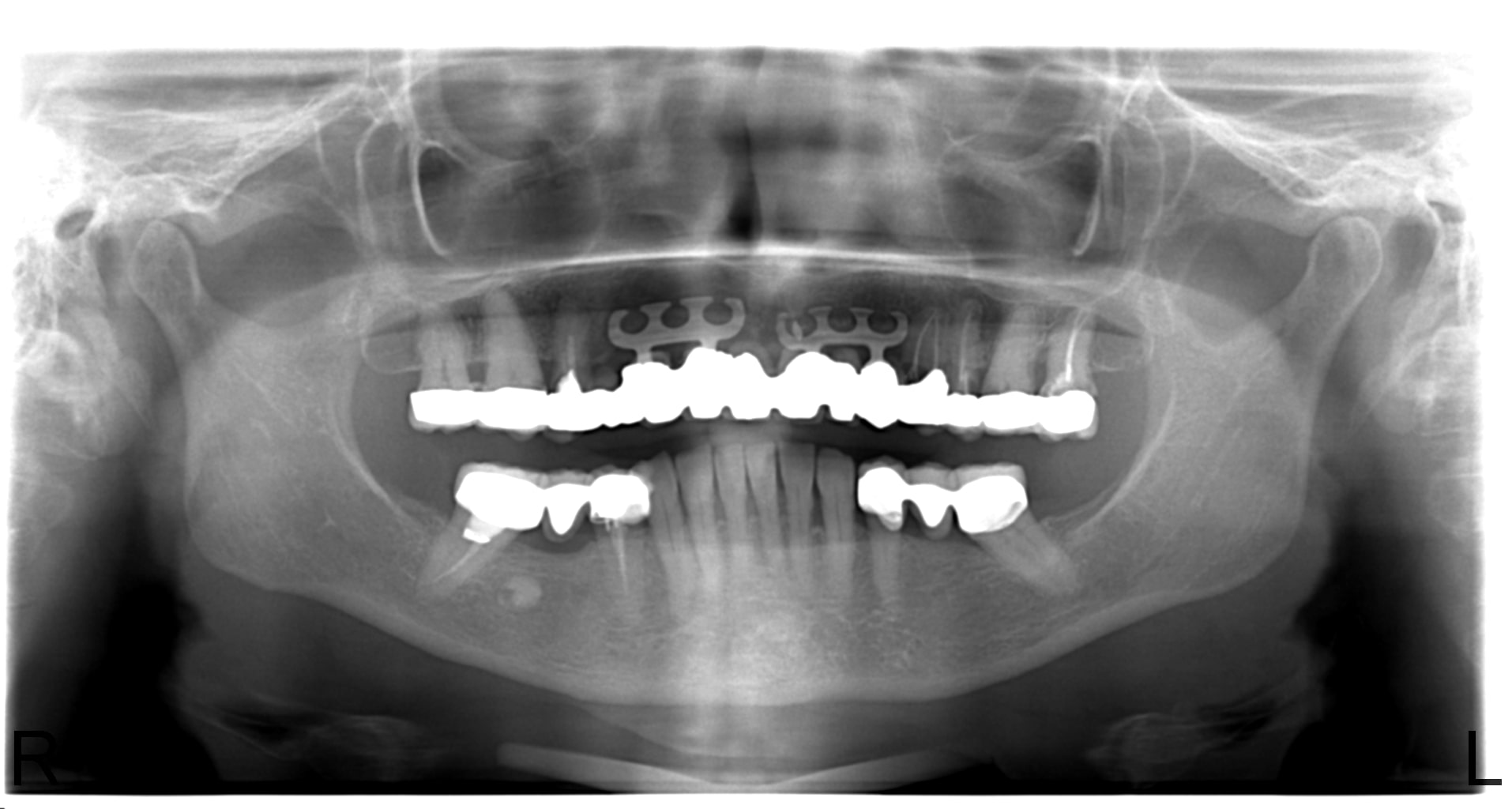
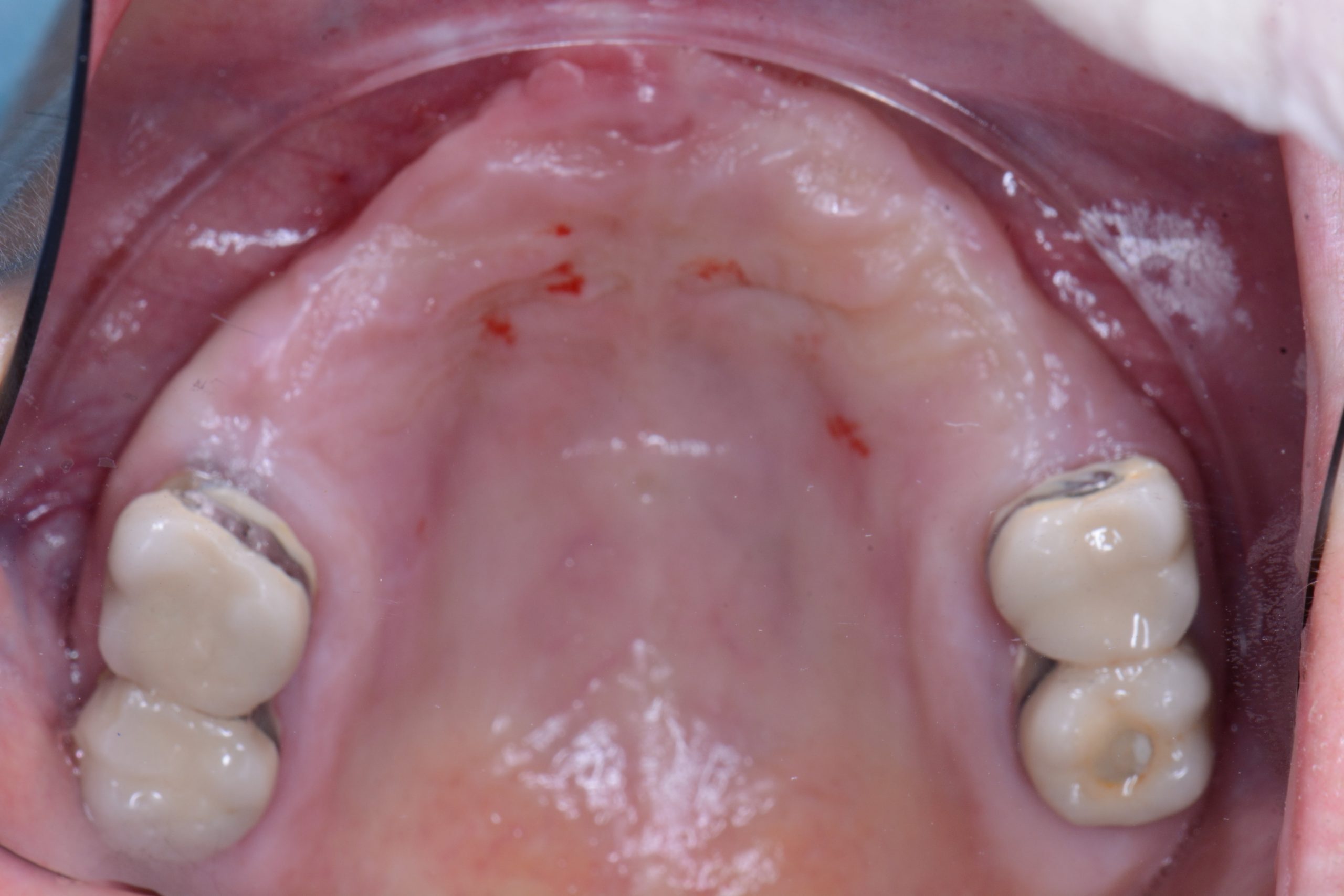
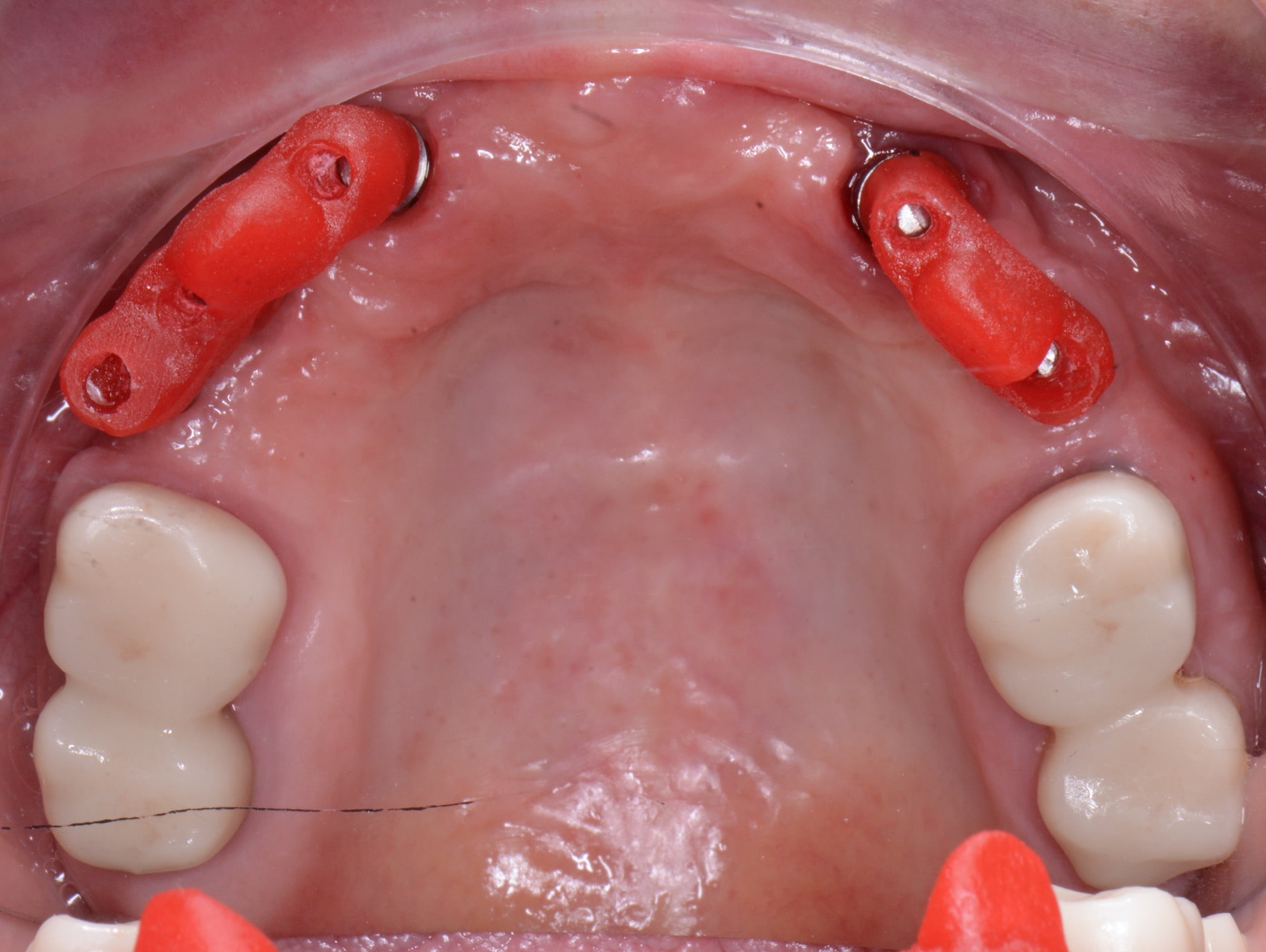
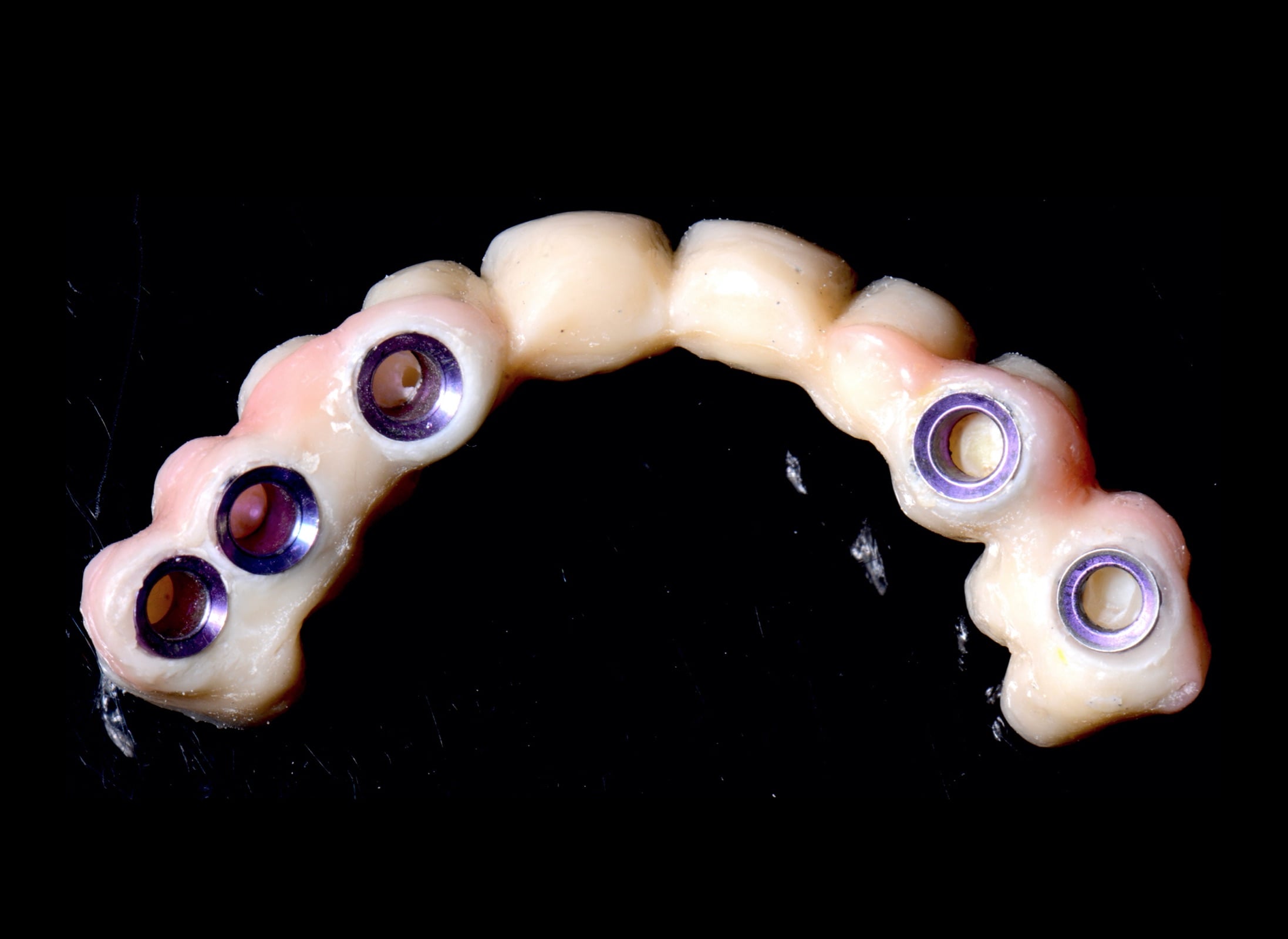
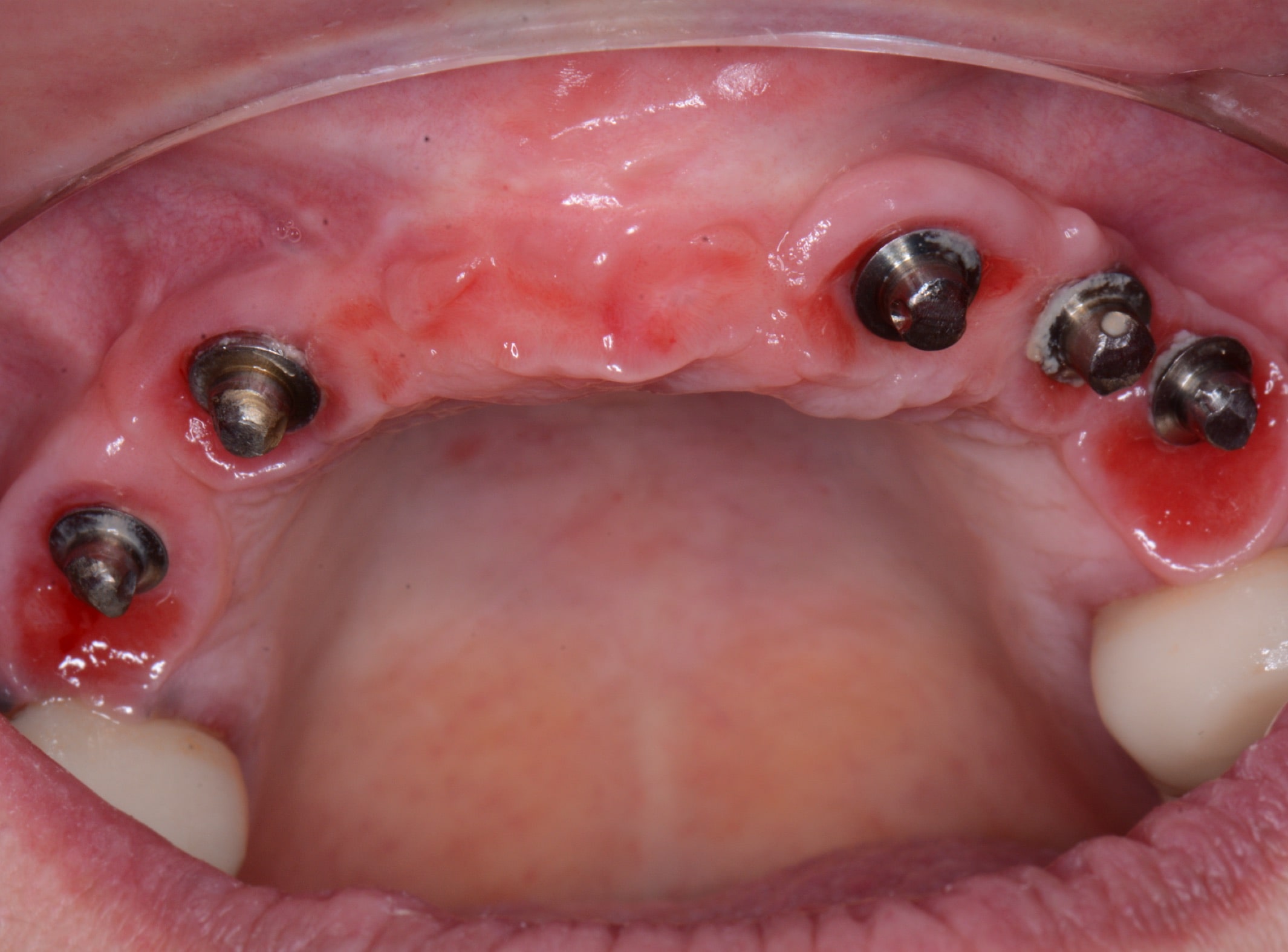
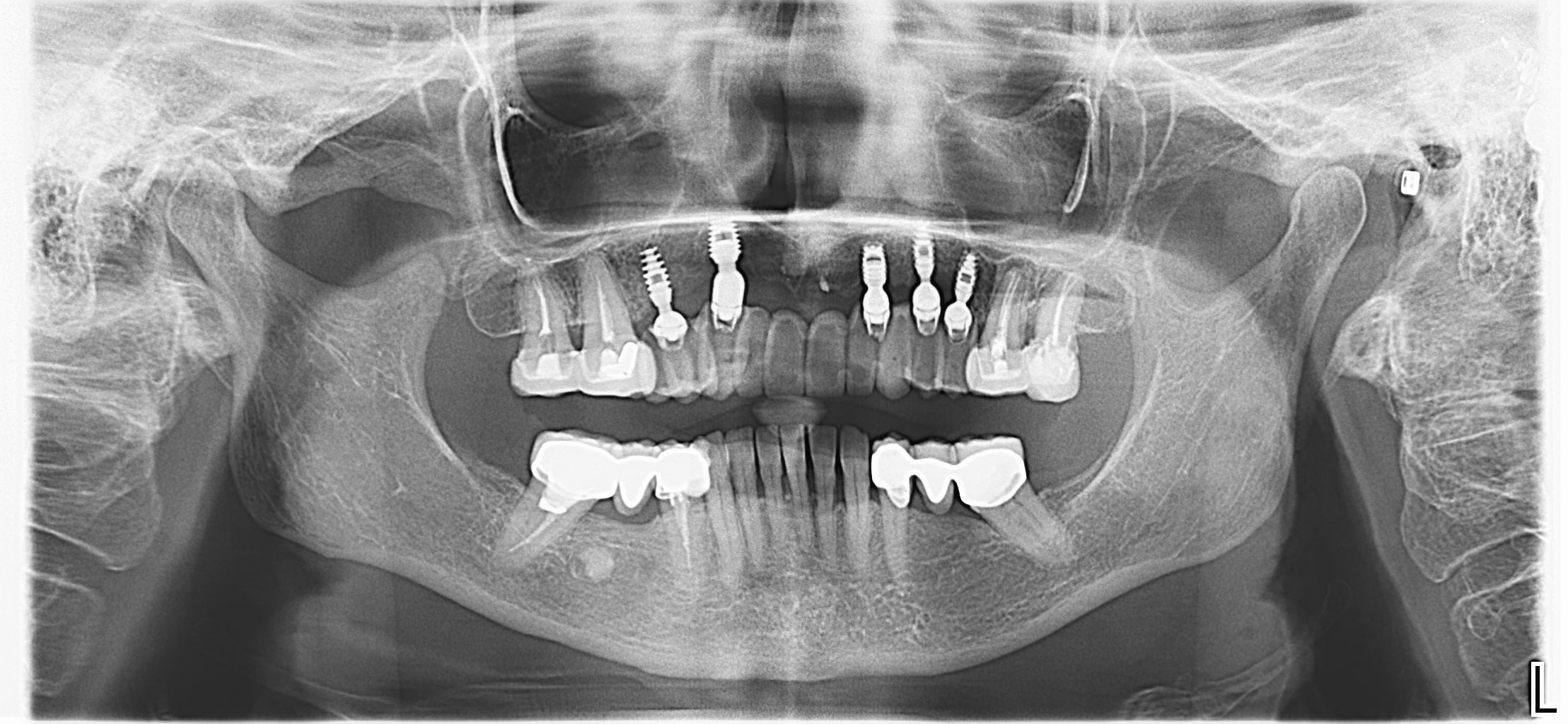
First, the definitive angulated abutments were placed on the implants using customized acrylic positioning keys (Figures 11 and 12) sent by the laboratory. In these abutments, a minimum angulation of around 3º should be chosen between them for optimal retention of the prosthesis. It is essential to keep this positioning key as it is the only way to be able to place the abutments in the same position. The copings (purple retentive or silver, non-retentive copings) were then placed on the abutments and checked to see that the gaps left in the frame BRWF did not interfere with the entry of the abutments and their copings. Once checked, a small amount of Vaseline was deposited inside the copings and left in place on the abutments, which facilitated the removal of the prosthesis after cementation. This ensured that the copings would not come off the abutments. The base of the prosthesis was also coated with Vaseline, and then the dual-cured cement was poured into the lodges of the copings at the base of the prosthesis. The prosthesis was positioned on the copings, and the patient was asked to occlude while the cement was being polymerized on all the copings simultaneously. Once the polymerization time had elapsed, the prosthesis was removed to eliminate any remaining cement and Vaseline (Figure 13) as well as any that remained on the abutments. Alcohol is also frequently used to eliminate humidity. Finally, the prosthesis was inserted again, which would not be removed until the next checkup. Note that although it appears to the patient that the prosthesis was fixed, the dentist, of course, will be able to remove it for maintenance. After a year of followup, the prosthesis was removed to clean it and evaluate the peri-implant soft tissues and the crestal bone level with several radiographs (Figures 14 to 17).
 |
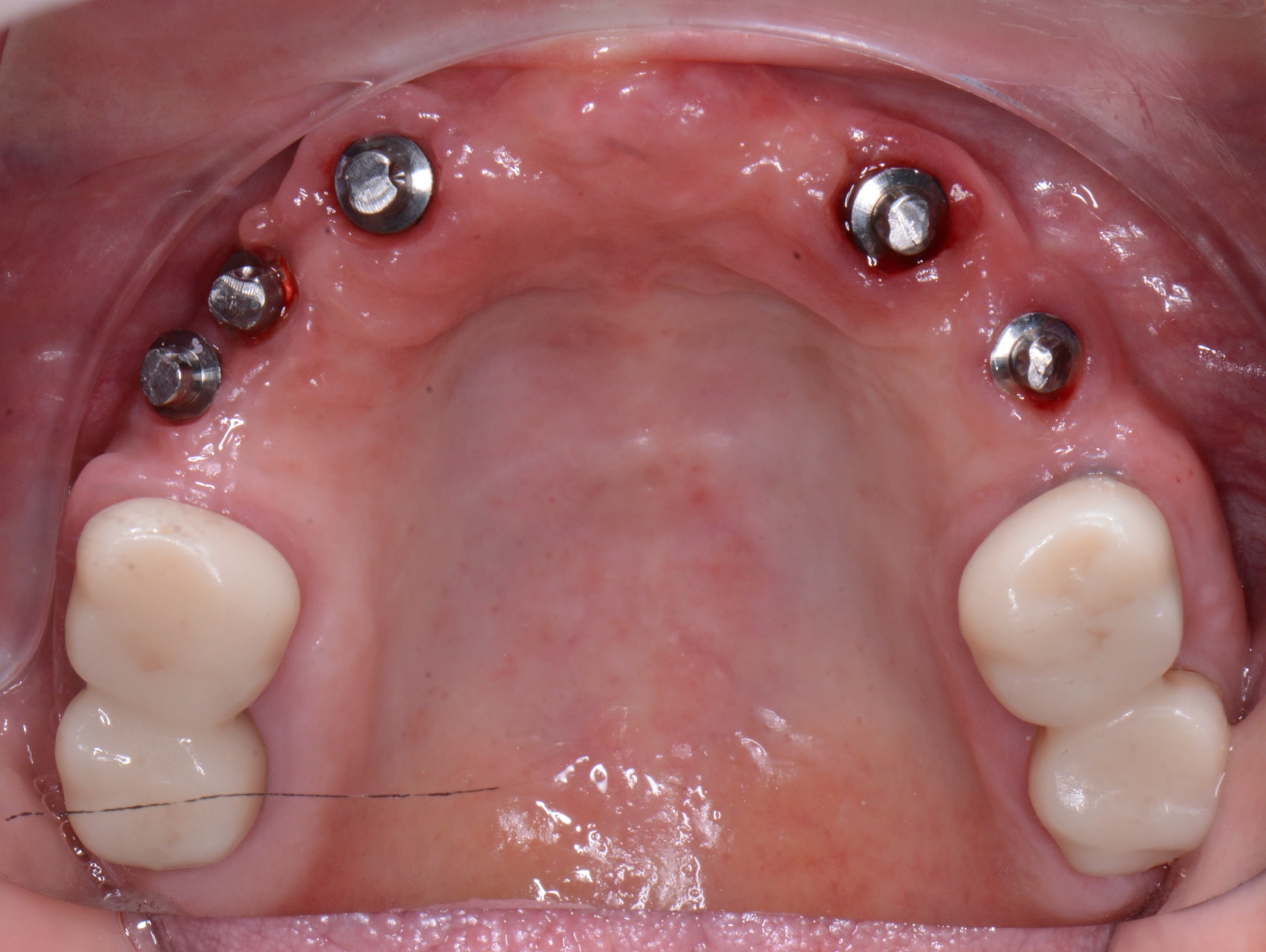 |
| Figure 11. Placement of the definitive abutments on the implants using an acrylic positioning key. | Figure 12. Abutments placed on implants. |
 |
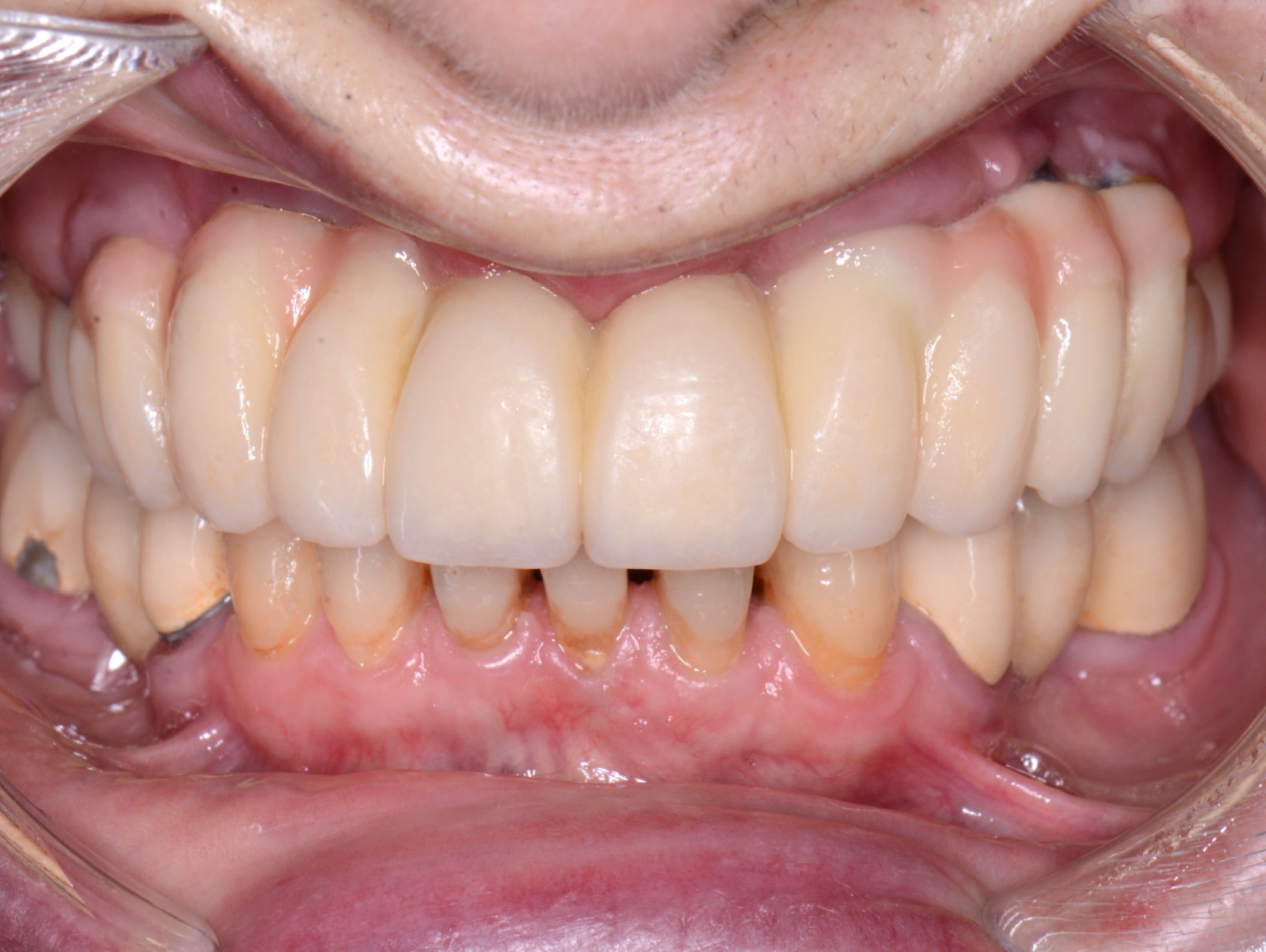 |
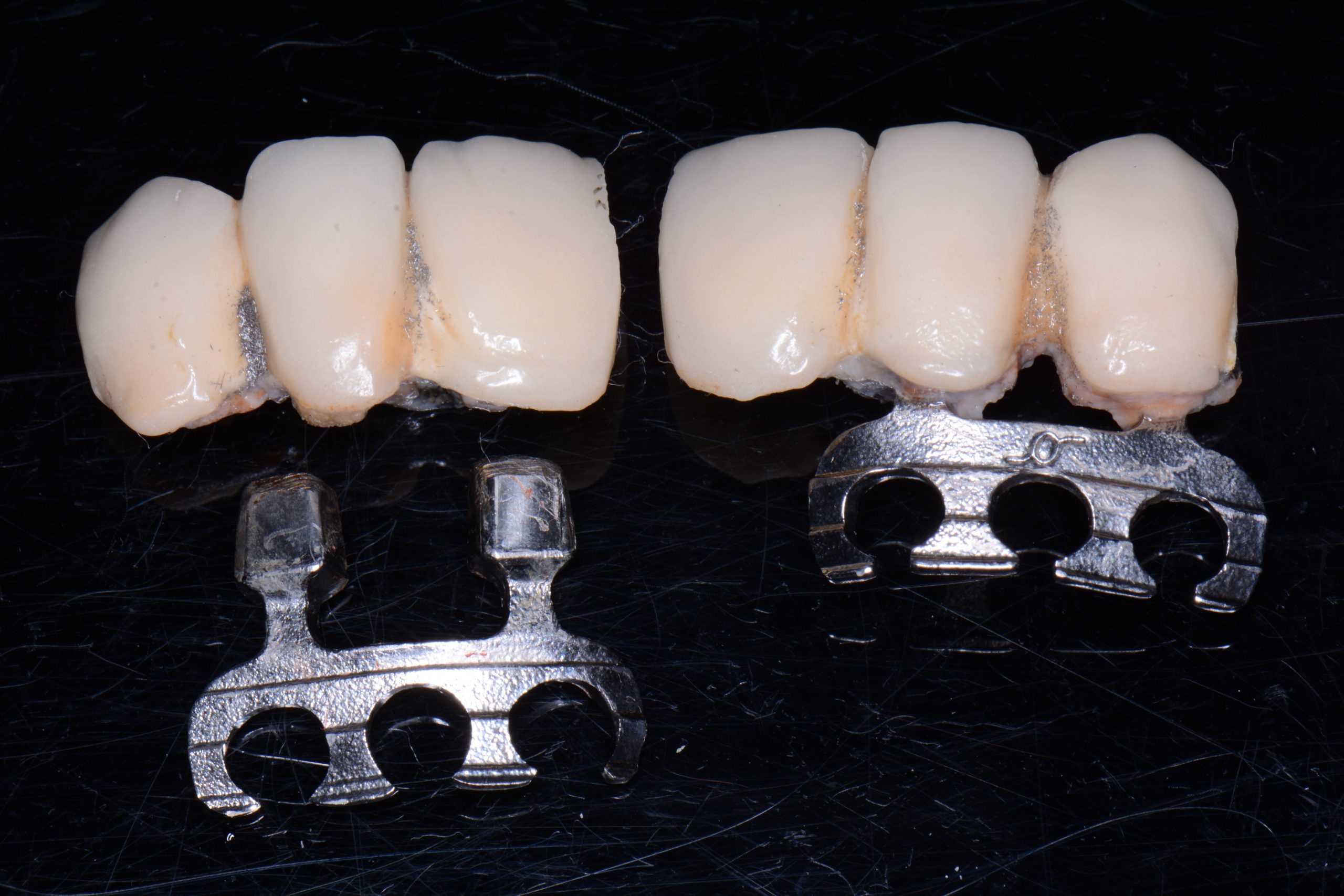
| Figure 13. Hybrid prosthesis with a substructure of biopolymer reinforced with fiberglass (BRWF) covered with ceramic, reinforced composite resin after cementing the copings. | Figure 14. Maxillary BRWF hybrid prosthesis after one year of followup. |
 |
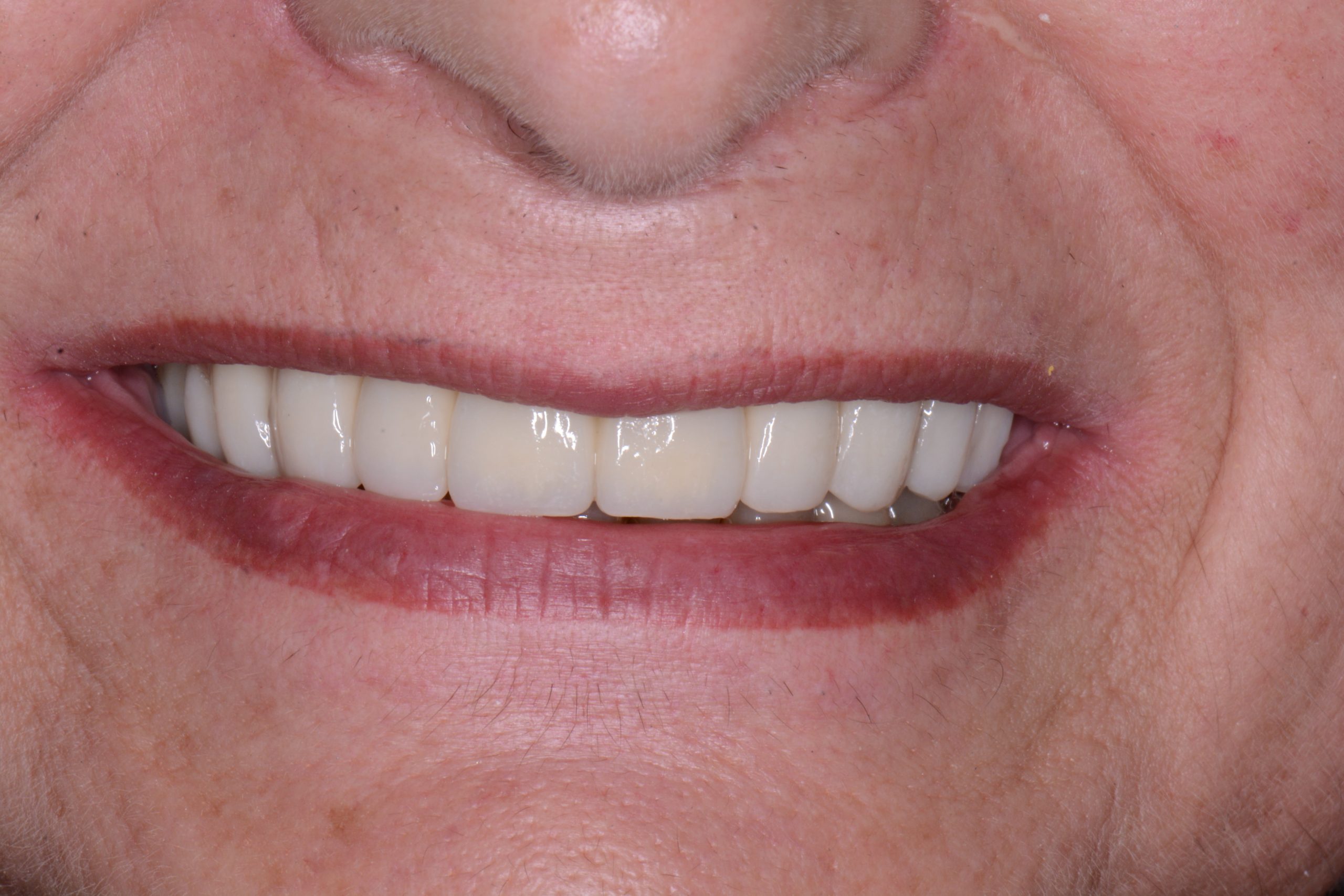 |
| Figure 15. Peri-implant mucosa after removal of the hybrid prosthesis at the one-year followup. | Figure 16. Aesthetic result. |
 |
|
| Figure 17. One-year follow-up OPG. |
DISCUSSION
The Bicon Dental Implants have a 1.50° Morse taper connection and a space between the implant and the abutment of less than 0.50 µm, which is a barrier to bacterial infiltration and also allows an adaptive position (0° to 360°) of the abutment.2 This is an implant system that dispenses with mechanical retention elements such as cement or screws, avoiding the complications associated with both types of retention systems since the crown is integrated into the abutment. The latter couples to the implants through friction with a conical seal, obtaining a great hold due to the elastic deformation between both parts, an effect known as “cold welding.”9 This union favors an excellent bacterial seal, avoiding the bad odors typical of other implant systems when the abutments are removed. In such a system, the joint surfaces must be dry as moisture can cause the abutment to come loose. This undesirable effect occurs more frequently in thick gingival biotypes or in those cases where the implant is positioned excessively subcrestal. The use of alcohol to clean the surface of the implant connection is recommended. These implants have a surface increased by 30% compared to[SC: that is 30% bigger than?] screwed implants of the same dimensions, which favors the formation of Haversian bone similar to the cortical bone between the spirals of its body.2 At the crestal level, due to the coronal design of the implant in the form of an inclined plateau, a “platform-switching” phenomenon is generated, which helps even more in bone formation.10
The prosthesis is a reinforced nanohybrid biopolymer with several layers of multidirectional glass fiber joined by epoxy resin adapted to modern CAD/CAM systems.2,3 Specifically, it is composed of 40% epoxy resin and 60% fiberglass.4 According to the manufacturer’s instructions, a minimum thickness of 0.70 mm for the biomaterial and 7 mm2 for the connector and a cantilever of no more than 15 to 18 mm are recommended. It is characterized as being a material with low specific weight, great bending strength (393 MPa), compression (374 MPa), and shear (339 MPa) and that is machinable and completely biocompatible.2,3 It has a modulus of elasticity similar to dentin (18.80 GPa and 12 to 14 GPa, respectively) which is more favorable than titanium (102 to 108 GPa)4 (Table 1).
BRWF is indicated as a material in temporary and/or definitive restorations; in the aesthetic or posterior sector; in single, partial, and complete rehabilitations; and even as substructures on natural teeth or implants.3,4 Usually, the substructure of BRWF is covered by indirect composite resin reinforced with ceramic 2,3,10 or, in a conventional way, by acrylic teeth and resin.10 In addition to its great versatility, this material reduces the impact force on the implants by approximately 50%, allowing good adaptation of patients3 and adequate preservation of the crestal bone.11 Another advantage is that it is very lightweight and can be used in patients with metal allergies since it is an inert material.1 In addition to offering better aesthetics and a lower cost and avoiding undesirable effects inherent to conventional materials, such as corrosion, repair is simplified. In the case of fracture or chipping, these prostheses can be removed and repaired extraorally. This ease of removal also allows the evaluation of peri-implant soft tissues and prevents/avoids the performance of regenerative procedures or the insertion of angled implants. Also, as it is an implant-borne prosthesis on short implants, it reduces the stress of the patients, the associated postoperative morbidity, and the total treatment time.11 Apart from all these advantages, these types of prostheses do not retain bacterial plaque like other alternative materials.3 In contrast, their main disadvantage is that the precise technology for manufacturing BRWF frameworks is complex and requires experience and adequate training from the clinician and the prosthetic laboratory.2
CONCLUSION
BRWF is a material with optimal properties for the manufacturing of metal-free substructures. Its adaptation to short implants enables the rehabilitation of patients with severe atrophies of the jaws, reducing the morbidity associated in these cases with regenerative procedures inherent to the insertion of implants of conventional lengths. Although it currently shows encouraging results, more studies are needed to evaluate its properties in the long term.
References
- Bradford-Smith P, Mitchel JC. How to use new materials and workflows to restore edentulous patients. Dental Products Report. 2019:42–4.
- Biris C, Sver-Bechir E, Bechir A, et al. Trinia reinforced polymer as core for implants superstructure. Mater Plast. 2017;54(4):764–7. doi:10.37358/MP.17.4.4941
- Biris C, Sever-Bechir E, Bechir A, et al. Evaluations of two reinforced polymers used as metal-free subestructures in fixed dental restorations. Mater Plast. 2018;55(1):33–7. doi:10.37358/MP.18.1.4958
- Ewers R, Perpetuini P, Morgan VJ, et al. Trinia: Metal-free restorations. Implants. 2017;1:22–7.
- Salgado-Peralvo AO. Historia clínica y exploración en Implantología Oral. Self-published; 2019.
- Atwood DA. Bone loss of edentulous alveolar ridges. J Periodontol. 1979;50:11-21. doi:10.1902/jop.1979.50.4s.11
- Romandini M, De Tullio I, Congedi F, et al. Antibiotic prophylaxis at dental implant placement: Which is the best protocol? A systematic review and network meta-analysis. J Clin Periodontol. 2019;46(3):382–95. doi: 10.1111/jcpe.13080
- Misch CE. Bone classification, training keys to implant success. Dent Today. 1989;8:39–44.
- Urdaneta RA, Marincola M. The Integrated Abutment Crown, a screwless and cementless restoration for single-tooth implants: a report on a new technique. J Prosthodont. 2007;16:311–8. doi:10.1111/j.1532-849X.2007.00194.x
- Marincola M, Morgan VJ, Perpetuini A, et al. Fixed full arch metal-free prosthesis on four short implants. African Journal of Dentistry and Implantology. 2013;1:34–7.
- Passaretti A, Petroni G, Miracolo G, et al. Metal free, full arch, fixed prosthesis for edentulous mandible rehabilitation on four implants. J Prosthodont Res. 2018;62(2):264–7. doi:10.1016/j.jpor.2017.10.002
Dr. Salgado-Peralvo is an associate professor of the Master’s in Family and Community Dentistry at the University of Seville and has a private clinical practice in Vigo, Spain. He has published more than 20 scientific articles and has written and edited 2 textbooks. He studied dentistry at the Complutense University of Madrid and studied a Master’s degree in Oral Implantology and Family and Community Dentistry at the University of Seville. He is currently developing his doctoral research at the University of Seville. He can be reached at orionsalgado@hotmail.com.
Dr. Salgado-García is a stomatologist who graduated from the Complutense University of Madrid. He is currently in private practice in Vigo. He is the author of several scientific publications and has collaborated on 2 textbooks. He was a dentist at the Galician Health Service (SERGAS) on leave and is a Medical Lieutenant Colonel of the Spanish Army in the Reserve. His postgraduate training includes university degrees in Oral Implantology, Oral Surgery, and Periodontics. He can be reached at asgvigo@hotmail.com.
Dr. Peña-Cardelles is a professor in the Master’s in Oral Surgery and Implantology at Rey Juan Carlos University, where he studied dentistry. He attained the title of Specialist in Oral Medicine at the Complutense University of Madrid, a Master’s degree in Pain and the Master’s degree in Oral Surgery and Implantology at Rey Juan Carlos University. He has developed and is developing his practice at a clinical and private hospital level. He has published more than 30 scientific articles concerning his field of specialization. He is currently developing his doctoral research on oral cancer at the La Paz University Hospital in Madrid and at Rey Juan Carlos University. He can be reached at juanfranciscopenacardelles@gmail.com.
Dr. Kewalramani is a professor in the Master’s in Advanced Implantology, Tissue Regeneration and Implant Rehabilitation at Rey Juan Carlos University. He studied dentistry and attained his Master’s degree in dental sciences at the Complutense University in Madrid and his Master’s in Advanced Implantology, Tissue Regeneration and Implant-Supported Rehabilitation at Rey Juan Carlos University. He has developed and continues developing his practice at a clinical and private hospital level. He is currently developing his scientific research at Rey Juan Carlos University. He can be reached at k93.naresh@gmail.com.
Gómez-Polo is an assistant profesor in the Department of Stomatology I (Orofacial Prosthesis), Faculty of Dentistry, Complutense University in Madrid, where he is also the director of the Universitary Specialist in Implant-Prosthesis. He can be reached at mgpolo@odon.ucm.es.
Disclosure: The authors report no disclosures.
Related Articles
Fully Guided Full-Arch Implant Reconstruction
Continued Innovation for Full-Arch Immediate Loading: Small Access Hole Technology
Guided Ridge Healing With Full-Arch Custom Prosthetics