
As facial injectable practitioners, we strive to deliver the safest, most comfortable aesthetic treatment results achievable, and cosmetic injectable treatments continue to increase year after year.
Not too long ago, these services were primarily performed to mitigate the effects of aging. What began as line-chasing and wrinkle relaxing has evolved into pan-facial rejuvenation aimed at achieving volumetric harmony.
In the age of social media, the injectable market has expanded to include a younger demographic with the goal of facial re-balancing. These treatments, aimed at enhancement and preservation of youth rather than correcting age-related changes, include chin augmentation, lip plumping, liquid rhinoplasty, and gummy smile modification.
Collectively, these procedures are known as profiloplasty. Consumer popularity of cosmetic injectable treatments can be attributed to their minimal downtime, quick results, lack of a need for general anesthesia, minimal to no pain, reasonable costs, and low occurrence of major side effects.
Successful soft-tissue filler treatment is predicated on proper patient selection, injectable product properties, placement precision, and, certainly, an esthetic eye. From the practitioner’s perspective, the primary concern is always safety.
Over the last decade of soft-tissue filler injections, the blunt-end microcannula has gained popularity among practitioners and patients alike. Patients are grateful for the decreased number of needle puncture sites, increased comfort, and decreased bleeding and bruising. Practitioners appreciate these same qualities, in addition to the commonly held belief that cannulae are safer and cannot penetrate vessels. But does science support this assumption?
As the soft-tissue filler market expands from restorative anti-aging treatments to multigenerational enhancements, the number of reported treatments increases annually. Unfortunately, but not unexpectedly, the number of adverse events rises synchronously.
 |
 |
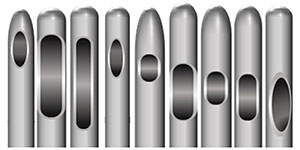
| Figure 1. Extrusion ports in microcannulae vary in location and size. | Figure 2. Microcannulae tips vary in shape. |
 |
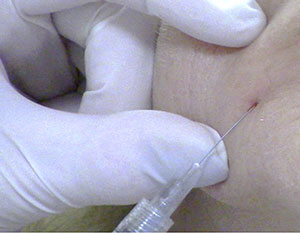 |
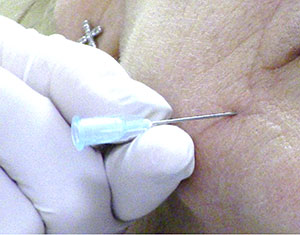
| Figure 3. An introducer (pilot) needle such as this 23-gauge needle can be used to create a portal in the skin for a 25-gauge cannula. | Figure 4. Once the portal is created, a 25-gauge blunt-end cannula can be introduced into the skin. |
The most dreaded and potentially serious complications are those that are vascular in nature, whereby dermal filler is introduced into an artery or vein. Vascular compromise is a known potential adverse event that has been observed since the dawn of cosmetic injectables in the early 1900s.
Much attention has been focused on this potentially devastating outcome over the last decade and how best to avoid it. To that end, the flexible and blunt-end microcannula has been suggested, and generally accepted, as a safer alternative to the hypodermic needle.
Cannula History
The word “cannula” comes from Latin, meaning “little reed.” Essentially, a cannula is a flexible tube that can be inserted into a bodily cavity, duct, or vessel to administer or remove fluid. Cannulae have been used for various applications in medicine for more than a hundred years, most frequently for intravenous therapy.
Any discussion about cannulae requires an understanding of gauge. Medical devices such as needles, catheters, suture wires, and cannulae are frequently standardized to the Birmingham Gauge System. This system is also known as the Stubs Iron Wire Gauge or the Birmingham Wire Gauge System.
These measurements can be confusing to the medical neophyte since their codification is counterintuitive. For example, a 25-gauge device is larger in diameter than a 32-gauge device. Understanding the system’s derivation helps make sense of this seemingly paradoxical system of classification.
When international trade became common, standardized wire diameters became necessary. Without a standard reference, ordering wire from another country would be risky and nearly impossible. Likewise, interchangeable parts couldn’t exist without an established scale.
Although their precise year of codification is uncertain, the unit measures associated with the Birmingham Gauge System were firmly established and in regular use before 1735. Although several wire measurement systems were in use simultaneously, the Birmingham method became the world’s adopted framework by which wire diameters would be referenced and standardized.
Understanding how wire is created explains the seemingly odd numerical classification. The process begins with the pulling of a solid metal rod through a conical shaped die. That first draw, and thereby the first reduction in diameter, is designated as 2 gauge. With each successive draw (3, 4, 5, etc), the wire caliber decreases in size.
The die aperture dimensions aren’t based on a desired mathematical width of wire. Rather, they’re based on the tolerance of the metal to the process of the wire drawing. The aperture must be narrow enough to decrease the wire diameter but wide enough for it to pass through without breaking.
Essentially, a balance has to be struck between meaningful reduction and wire destruction. Therefore, a gauge represents the number of passages a rod has made through a known sequence of dies. This explains the antithetical nomenclature of larger numbers associated with smaller sizes.
It should be obvious then that gauge is a measurement of the external diameter of a cannula. The width of the internal lumen, or hollow, of a cannula is a function of the wall thickness, which differs with each manufacturer. Other production variables include the location and orientation, the size and number of the side extrusion ports (Figure 1), the length and flexibility of the shaft, and the shape of the cannula tip, which can be oval, rounded, or rounded trapezoidal (Figure 2).
Microcannula Variation
Blunt-end cannulae have been used for autologous fat transfer to restore volume since the early part of the last century.1,2 These fat grafting cannulae are large, with internal diameters ranging from 2 to 4 mm. This allows for the viability of adipocytes as well as adipose stem cells required for graft retention. Much smaller cannulae became available in the early part of this decade, paving the way for intradermal use with soft-tissue fillers such as hyaluronic acid.
Numerous microcannulae cleared by the Food and Drug Administration (FDA) are manufactured and distributed by various companies. According to the American Society for Testing and Materials (ASTM), the standard tip of cannulae (and chemical requirements for stainless steel) comprises similar but not identical combinations of carbon, manganese, phosphorus, sulfur, silicon, chromium, and nickel.
As stated, the tip shape variations are oval, rounded, or rounded trapezoidal. Cannula flexibility is a result of the proportional combinations. Studies indicate that flexibility can vary between cannula up to a factor of two. However, regarding vascular penetration, all parameters including modulus of elasticity, tensile strength, tenacity, abrasive and corrosive properties, and biosecurity are irrelevant when the cannula gauge falls below 27. Research demonstrates that these smaller-gauge cannulae require the same force as an equivalent gauge needle to penetrate vessels.
Cannulae in Aesthetic Medicine
Intentionally designed without a cutting edge, cannulae require a portal before they are introduced into the soft tissue. A pilot or “introducer” needle is used for the initial skin penetration. Ideally, the pilot needle should be a gauge larger than the cannula. For instance, a 23-gauge needle is used to introduce a 25-gauge cannula to breach the skin (Figure 3).
The pilot needle is inserted in a 30° to 45° angle to the skin and directed in what will be the desired cannula path. The depth of needle insertion should be at least the length of the bevel but not so deep as to cause unnecessary trauma and bleeding.
If placed too superficially, though, the cannula will meet resistance, resulting in pain and multiple failed insertion attempts. A 360° rotation of the needle is helpful to ensure access patency. The needle should remain in the skin for a few moments before removal, creating a smooth channel that allows ease of cannula passage (Figure 4).
Microcannulae interest and use has escalated since their debut in the United States injectable market in 2008. The prevailing opinion was that, due to their blunted end, cannulae were safer than needles when injecting dermal fillers. This conclusion that inadvertent vascular injection risk could be eliminated without a sharp leading edge was a widely held belief.
This theory, unfortunately, was an untested assumption. Very soon after their introduction, evidence contrary to this premise emerged. It became clear that cannulae were indeed capable of vascular penetration, resulting in dangerous occlusive and embolic events.
By 2015, the growing number of these disastrous episodes led to multiple evidence-based studies confirming what practitioners saw in practice. Cannulae can and do penetrate vessels. Sadly, many of today’s practitioners are unaware of these findings and believe vascular events are avoidable with cannulae.
Microcannulae have many qualities that make them desirable, but they are not without risk, and size plays a dominant role in these potential dangers. In fact, over the last five years, leading academic and industry investigators have advocated the elimination of 30-gauge cannula as a safety strategy. It is recognized that these smaller gauge cannulae freely penetrate vessels in the same manner as needles of the same gauge. Consequently, this well documented hazard has led to total exclusion of 30-gauge cannulae in more recent investigations.
Evidence proving cannulae smaller than 25 gauge behave exactly the same as needles of the same gauge with respect to vascular penetration continues to accrue. One 2019 study including cannulae and needles in the 27- to 20-gauge range demonstrated that a 27-gauge cannula can penetrate arteries with the exact same force as a 27-gauge needle. It determined that the force required to penetrate an artery decreases in a “statistically significant fashion” with smaller-diameter instruments. This is true for both needles and cannulae.
The study also confirmed earlier data indicating that cannulae 25 gauge and larger are capable of penetrating vessels. However, these larger-diameter cannulae require significantly more force for penetration than a same-sized needle. The experiment further revealed that the force required to puncture a vessel decreases with the age of the patient.
One of the strengths of this study was its use of non-embalmed and non-latex injected specimens. This is important, as it is widely known that postmortem vascular injection of latex significantly influences the amount of force needed to penetrate vessels.
Without question, microcannulae offer some technical advantages over traditional needles. The ability to deliver broad, multidirectional threads and aliquots of filler with fewer needle insertion sites is a distinct benefit. But if safety is defined as the inability to penetrate vasculature, it is clear that 27-gauge and smaller cannulae are not superior to equivalent gauged needles.
There are many instances when needles are more desirable for product delivery than cannulae. For instance, areas requiring precise placement in small volumes such as oral commissures and lips benefit from the ease of accuracy offered by a needle.
Although many practitioners switched from needles to cannulae based on the premise of safety, this assumption is no longer supported. Safety guidelines now endorse the more exacting method of a needle instead of the 27-gauge or 30-gauge cannula. This favors a return to the greater precision afforded by a needle in situations when a 30-gauge or 27-gauge cannula substitution was based solely on safety concerns.
The field of facial injectables continues to evolve with products and standards conforming to scientific advances. Protocols and guidelines related to every aspect of the field change and are updated annually. Moreover, a technique or device is not acceptable simply because others in the industry use it.
In 1979, the FDA banned the use of liquid silicone as a dermal filler. Despite this official regulation and the fact that its cosmetic use is illegal in many states, some medical practitioners continue to perform these injections. It is important for practitioners to remain current and not slip below the community standard.
With respect to microcannulae, the research is in agreement. Cannulae that are 27-gauge and smaller are no less likely to penetrate vessels than needles of the same gauge. In terms of safety, industry experts recommend the use of 25-gauge or larger cannula when delivery with a needle is not essential to the aesthetic outcome.
Dentistry and all healthcare is beholden to evidence-based data when ministering to patients. This is called the standard of care. On the subject of vascular penetration by cannulae, that evidence is clear.
In 2016, Goodman et al reported that in 17% of the cases in which an intraarterial injection of dermal filler occurred, the injection was performed using a cannula. Practitioners and patients alike must be made aware of the potential risks, and these risks are not eliminated simply by using a cannula.
References
- Miller CG. Cannula Implants and Review of Implantation Techniques in Esthetic Surgery. Chicago: Chicago Oak Press, 1926.
- Coleman SR. Structural fat grafts: the ideal filler? Clin Plast Surg. 2001;28(1):111-119.
Dr. Meinecke has been active in the field of facial injectables since 2004. Her organization, FACES, provides cosmetic and therapeutic injectable training for medical and dental professionals with a core focus on comprehensive facial anatomy. Her courses integrate cadaver study with live patient injections. In addition to teaching nationally, Dr. Meinecke is the facial injectables course director and instructor at the Boston University School of Dental Medicine. She is the author of Start and Grow Your Cosmetic Injectable Practice and a past president of the Maryland Academy of General Dentistry. She is a past chair of the ADA Council on Communications and serves as a spokesperson for the Academy of General Dentistry (AGD). She is a Fellow in the AGD, the International College of Dentists, and the American College of Dentists as well. She maintains a private practice in Potomac, Maryland.
Related Articles
Should You Add Facial Injectables to Your Practice?
When and How to Say No to Facial Injectable Patients
The Lower Face Influences Total Aesthetics in Injectable Procedures











