As a full-time educator, I am happy to report that dental education is teaching current didactic and clinical endodontic information. Unfortunately, this was not the case when I graduated from dental school. Back then, dental education seemed to be teaching what historically had been taught for years rather than addressing the current thought and clinical practice of the times.
In part 1 of this article, I discussed diagnosis, anesthesia, and instrumentation in endodontics. In the final part of this article, I will discuss obturation, the use of medication, and the endodontic-restorative relationship.
OBTURATION
The purpose of obturation is to eliminate all avenues of leakage from the oral cavity or the periradicular tissue into the root canal system. Also, obturation should help seal in any irritants (eg, necrotic tissue) or pulp tissue that could not be fully removed during canal cleaning and shaping procedures so they do not leak out of the root canal system and cause breakdown of the periradicular tissues.1 Dental school taught us lateral compaction of gutta-percha. This method is consistent, easy to learn, and when problems arise, they can be corrected.
A study by Brothman2 demonstrated that there was no statistical difference in filling efficacy when warm vertical and lateral compaction of gutta-percha were compared in cross sections. However, radiographic examination of these specimens revealed that the warm vertical compaction technique had nearly doubled the number of lateral and accessory canals. Also, warm vertical compaction produced a denser endodontic filling than the lateral gutta-percha compaction technique.
Since we think about tapered canal preparations in 3 dimensions, the same must hold true for obturation. The question is, Why switch from lateral compaction when it has stood the test of time and is a very consistent technique? The reason is that warm gutta-percha better adapts to the 3 dimensions of the tapered root canal systems that we are preparing today. Canal preparation techniques we learned in dental school were conservative in comparison, and lateral gutta-percha compaction met the goal of sealing these root canal systems.
When compared with warm vertical gutta-percha compaction, lateral compaction is more ergonomically difficult to perform when obturating tapered canal preparations. The tapered canals require an increase in the amount of accessory cones needed to laterally fill a canal, thus making it difficult for the clinician to visualize. Also, the lateral forces create sealer flow more coronally rather than apically. This is why Brothman’s2 study demonstrated the minimal number of lateral and accessory canals filled with lateral compaction compared with vertical warm gutta-percha compaction.
There are many types of thermoplastic gutta-percha delivery systems on the market. While certain systems may considerably shorten the time needed to obturate a canal when compared with lateral compaction, the clinician must be aware of the following disadvantages that may exist: (1) there may be no margin of error with placement; (2) there may be a greater propensity to overfill; and (3) it can be difficult to re-treat.
 |
 |
| Figure 1. Tapered gutta-percha cones (DiaDent). | Figure 2. Kerr EWT sealer. |
 |
 |
| Figure 3. System B heat source (Sybron Endodontics). | Figure 4. Obtura for warm gutta-percha backfill. |
 |
| Figure 5. Luks pluggers (Pulpdent Corp). |
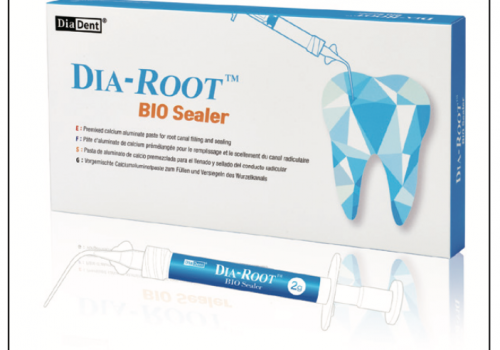
The obturation technique used by the majority of the estimated 4,500 endodontists in the United States is warm vertical compaction. A 0.6- or 0.4-taper gutta-percha cone (Figure 1) is calibrated to the size and taper of the master apical file (the largest file placed to working length). Kerr EWT sealer is recommended and should be mixed to break approximately one inch off the mixing slab (Figure 2). The sealer is a zinc-oxide eugenol cement, and when it sets, it forms zinc eugenate, a prostaglandin inhibitor. Prostaglandins are known pain mediators of the body’s inflammatory response (discussed further in the pain control section).3 This is the reason that, although overfills will occur with vertical warm gutta-percha compaction, the patient will not have an increase in postoperative flare-ups. The System B (Sybron Endodontics, Figure 3) is used as a heat source, and the Obtura (Obtura Corp, Figure 4) is used to backfill the canal with warm gutta-percha after the master gutta-percha cone is seared with the System B. Luks pluggers (Pulpdent Corp, Figure 5) are used to down-pack the master cone after searing and backfilling with warm gutta-percha. The Luks plugger tip should be wiped with alcohol prior to placement in the canal to prevent it from sticking to the gutta-percha.
PAIN CONTROL MEDICATION
Although pain perception of root canal treatment by the general public has become magnified by the media, in reality the incidence of postoperative pain is relatively low. Georgopoulou4 reported that 78% of patients have no or slight pain after endodontic treatment. The most consistent factor that predicts postoperative endodontic pain is the presence of preoperative hyperalgesia.5 The physical removal of inflamed or infected pulpal tissue will greatly reduce a patient’s discomfort level.
When drugs are needed for pain control, it is important to understand that a dentist must balance a patient’s pain against the side effects of a particular drug. In dental school years ago, we were taught that narcotics were the best method of pain control in endodontics. In the last decade, clinical research has changed our previous educational thought on this subject. It is understood today that most of the analgesia in endodontic therapy is due to the effects of nonnarcotic medication (ie, NSAIDS–nonsteroidal anti-inflammatory drugs). Also, most side effects (nausea, vomiting, constipation, and respiratory depression) result from the use of narcotics. A clinician must not fall into the habit of always prescribing the same analgesic for all patients. Use a maximal dose of nonnarcotic medication first, then use the narcotic as an “adjunct.” This combination of drugs will increase analgesia but at the cost of increased side effects.
A common NSAID used in endodontics is ibuprofen. It has good oral absorption but is slowed with food. The recommended dosage is 600 mg every 6 hours with a 2,400 mg/24 hours maximum dose. It is important to note that ibuprofen has a ceiling dose of 600 mg. This means that anything over this amount may not have an additive effect for the patient. Other NSAIDs on the market are Anaprox, Lodine, and Orudis. Each of these drugs has anti-inflammatory action, but this varies as to the exact dosage and side effects.
 |
| Figure 6. Cyclooxygenase enzyme pathway. |
The goal in endodontic pain control is to prevent the pain cycle from occurring. NSAIDs inhibit arachidonic acid from breaking down into prostaglandins. This is biochemically referred to as the cyclooxygenase enzyme pathway (COX-1) (Figure 6). Prostaglandin is a chemical mediator that is not stored in tissue but rather is created at the site of inflammation to aid in the body’s inflammatory cycle. Prostaglandins increase pain due to this activation of the body’s inflammatory response. Studies have shown that NSAIDs given prior to treatment will reduce postoperative pain.6
Recently, there has been much discussion of the COX-2 enzyme pathway and COX-2 inhibitors Vioxx and Celebrex for use in medicine and dentistry. Nakanishi et al7 demonstrated that COX-2 levels increase in inflamed pulp when compared with normal pulp tissue. Research is ongoing to determine the effectiveness of COX-2 inhibitors in endodontics.
Narcotics work on the opiate receptors that are located in the brain and on peripheral nerves. There is a minimal amount of narcotic needed for clinically significant analgesia to occur. This dosage is 60 mg of codeine (2 tabs of Tylenol III) or 7 to 10 mg hydrocodone (1 tab of Vicodin), or 5 to 6 mg oxycodone (1 tab of Percocet). It is important to remember that opiates are good analgesics but not good anti-inflammatory medications.
A good medication to use if opiates are needed is Vicoprofen (200 mg ibuprofen and 7.5 mg hydrocodone). When prescribing this drug, the dentist must have the patient supplement this dose with 400 mg of ibuprofen because 200 mg is not at the effective ceiling dosage.
Acetaminophen is used in patients who cannot take NSAIDs. Like opiates, acetaminophen has little if any anti-inflammatory effect. It will provide analgesia and antipyretic effects similar to aspirin. Acetaminophens are 90% to 95% metabolized in the liver. Four percent of this dose is metabolized into toxic metabolites. This is the reason high doses and long-term use may cause liver damage. One main advantage of acetaminophen is that it has no real effect on blood platelets.
In cases when the clinician finds it difficult to control endodontic postoperative pain, systemic steroids can be administered. The advantage of steroids is that they block the breakdown of membrane phospholipids into arachidonic acid (Figure 6). The use of dexamethasone (Decadron), a synthetic adrenocortical steroid, has been recommended.8 This steroid medication should be prescribed as follows: Decadron 0.75 mg, disp: 9 tabs, Sig: day 1: 3 tabs stat, then 1 tab tid, day 2: 2 tabs bid, day 3: 1 tab. It is important to note that 1 day is 24 hours, so if you were to prescribe this at the end of the day, the patient does not need to ingest the first 6 tabs before going to bed that evening. Also, if there is an infection involved, place the patient on antibiotics, because steroids can block or mask the body’s response to infection.
Antibiotics
The goal of antibiotic therapy is to use an antibiotic that best targets the bacteria that is the etiology of an infection. Years ago, the field of endodontics treated infections of pulpal origin without having a good idea of the bacteria involved. Recently, with advanced culturing techniques, we better understand the microbiological aspect of endodontic infections.
Endodontic infections are polymicrobial, with a predominate amount of anaerobic bacteria cultured from these infections.9 Antibiotics are an adjunct therapy to endodontic treatment. Pen VK is the drug of choice. It inhibits the synthesis of the bacterial peptidoglycan cell wall. It is effective against gram-positive and gram-negative bacteria. A study by Vigil and colleagues10 demonstrated that the Pen VK spectrum of microbial activity includes many of the bacteria isolated in endodontic infections. The recommended dosage of Pen VK is 500 mg, disp: 22 tabs, Sig: 2 tabs stat, then 1 tab tid until finished.
The second antibiotic of choice is clindamycin. This antibiotic works well against anaerobic bacteria. It distributes well within bone, therefore it enhances treatment of osseous infections. Clindamycin is beta-lactamase-resistant (unlike Pen VK, which can develop bacterial resistances) and has a good spectrum against gram-positive and gram-negative bacteria. The recommended dosage is clindamycin 150 mg, disp: 22 tabs, Sig: 2 tabs stat, then 1 tab tid until finished. Clinicians can double this dosage if necessary. Gilmore et al11 compared Pen VK with clindamycin in treating odontogenic infections. The results demonstrated that both antibiotics produced similar results in treating oral infections.
One of the reasons dentists may not prescribe clindamycin is the chance their patients will develop pseudo-membranous colitis. It is important to note that this can occur with any type of antibiotic prescribed. Pseudomembranous colitis rarely occurs with the recommended oral endodontic antibiotic dosages. Its occurrence is usually seen in patients who are on intravenous doses of clindamycin in the hospital. Clinical signs and symptoms of pseudo-membranous colitis are abdominal pain, diarrhea, fever, and blood or mucus in stools. The treatment when this occurs is prescribing vancomycin or metronidazole.
A third choice of antibiotics for endodontic infection is metronidazole (Flagyl). This drug is very effective against obligate anaerobic bacteria. It distributes throughout the total body water. In endodontic usage, metronidazole is usually prescribed in conjunction with Pen VK (if ineffective after 2 to 3 days). This antibiotic combination provides a better spectrum against endodontic infections. The recommended dosage is metronidazole 500 mg, disp: 22 tabs, Sig: 2 tabs stat, then 1 tab tid until finished. Metronidazole is contraindicated in pregnant patients, and it should not be combined with alcohol during usage and for at least 3 days after the discontinuance of the medication.
The old school of thought involving endodontic antibiotic application was the use of erythromycin as a second choice if a patient was allergic to Pen VK or if Pen VK was not effective during treatment. Erythromycin is ineffective against many of the anaerobic bacteria involved in endodontic infections. Also, dentists should not use cephalosporins instead of Pen VK. This antibiotic has poor bone penetration and is ineffective against severe anaerobic infections. Cephalosporins have a 5% to 10% cross-reactivity with penicillin-allergic patients.
An antibiotic regimen in endodontic treatment should be 7 to 10 days of around-the-clock treatment. A patient should begin to respond to antibiotic therapy within 24 to 48 hours. The dentist should be in close contact with a patient on antibiotics. The clinician must be aware if the antibiotic is ineffective and hence the possibility of worsening clinical symptoms for the patient or allergic reactions to a particular antibiotic. Patients using oral contraceptives should be warned that the interaction of antibiotics can reduce the effectiveness of contraception.12
THE ENDODONTIC-RESTORATIVE RELATIONSHIP
The relationship between endodontics and restorative treatment is one of the most important interdisciplinary relationships in dentistry. Unfortunately, dental schools never reinforced the poor success rate with endodontic treatment when restorative treatment was not completed in a timely manner. The best endodontically prepared and obturated tooth will ultimately be doomed to failure if a proper final restoration is not completed. However, the best prosthodontically prepared tooth can ultimately have an effect on the pulp, causing inflammation or necrosis. I remember being taught in dental school that after I obturated a tooth, I should place a temporary restoration, then wait a few weeks before the final restoration is fabricated. It was thought that in case the patient had problems with the root canal, the removal of the temporary for possible endodontic re-treatment would be easier.
Today, it is better understood that coronal leakage is one of the main causes of endodontic failure,13 and obturating teeth that are asymptomatic at the fill appointment is not likely to result in flare-ups. At the time of obturation the dentist should consider restoring the endodontically treated tooth with post and core, core alone, or placement of a permanent restoration while the rubber dam is still in place. This will help prevent saliva leakage and ensure proper sealing of the endodontic fill. The built-up tooth can then have a permanent crown fabricated at a subsequent appointment.
Kim’s14 research on the neurovascular interaction of the pulp with injurious agents (mechanical, chemical, or bacterial) demonstrated the pulp’s limited ability to respond and heal itself from the effects of these agents. One of the main reasons that the pulp is limited in its healing ability is that the vascular system exists in a low-compliance environment. The hard tissue surrounding the pulp can only increase its blood flow approximately 2 times the normal amount, whereas other body organs can increase blood flow up to 10 times their normal amount. Remember, it is the blood that carries many of the inflammatory mediators that help the body fight inflammation and infection. Other low-compliance vascular systems in the body are found in the brain, fingernail beds, and bone marrow.
A simple tooth preparation for a crown or filling can cause the pulp to degrade from vital to necrotic over a period of time. This is why it is important to obtain a preoperative pulp vitality reading before any restorative treatment is performed. This provides the dentist with a better understanding of the pulpal status. If a patient’s pulp tests within normal limits preoperatively, and if after a crown prep is completed the patient complains of lingering pain to cold, it is wrong to assume that cementing the permanent crown will resolve the patient’s pain. Rather, this is a classic example of neurogenic inflammation due to the pulp’s inability to resolve the inflammation caused by the mechanical stimulation. The treatment choice is endodontic therapy prior to placement of the final restoration. This case scenario is one of the reasons why I recommend to dentists performing restorative treatment on asymptomatic vital teeth that they inform their patients prior to starting the restorative therapy of possible nerve involvement during or after treatment. It is important to note that many pulps respond just fine to restorative treatment, and not every tooth needs endodontic treatment before or during restorative treatment.
CONCLUSION
I have learned a lot about the field of endodontics since dental school. The technological advances in thought and clinical application have been significant in the last 8 to 10 years. I better understand that microbial or inflammatory etiology of pulp tissue is the primary cause for endodontic treatment. Also, the failure to eliminate these factors through proper “chemo-mechanical” canal preparation, not preventing further contamination of the periradicular tissues with a 3-dimensional obturation, in conjunction with lack of a permanent restoration after obturation, will ultimately lead to failure of conventional endodontic treatment.
References
1.Gutmann J, Witherspoon DE. Obturation of the cleaned and shaped root canal system. In: Cohen S, Burns RC, eds. Pathways of the Pulp. 8th ed. St Louis, Mo: Mosby; 2001.
2. Brothman P. A comparative study of the vertical and the lateral condensation of gutta-percha. J Endod. 1980;7:27-30.
3. Nguyen N. Obturation of the root canal system. Pathways of the Pulp. 6th ed. St Louis, Mo: Mosby; 1994.
4. Georgopoulou M, Anastassiadis P, Sykaras S. Pain after chemomechanical preparation. Int Endod J. 1986;19:309-314.
5. Hargreaves K, Hutter J. Endodontic pharmacology. In: Cohen S, Burns RC, eds. Pathways of the Pulp. 8th ed. St Louis, Mo: Mosby; 2001.
6. Jackson DL, Moore PA, Hargreaves KM. Postoperative nonsteroidal anti-inflammatory medication for the prevention of postoperative dental pain. J Am Dent Assoc. 1989;119:641-647.
7. Nakanishi T, et al. Immuno histochemical analysis of cyclooxygenase-2 in human dental pulp. J Endod. 2001;27:385-8.
8. Krasner P, Jackson E. Management of posttreatment endodontic pain with oral dexamethasone: a double-blind study. Oral Surg Oral Med Oral Pathol. 1986;62:187-190.
9. Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427-430.
10. Vigil GV, Wayman BE, Dazey SE, et al. Identification and antibiotic sensitivity of bacteria isolated from periapical lesions. J Endod. 1997;23:110-114.
11. Gilmore WC, Jacobus NV, Gorbach SL, et al. A prospective double-blind evaluation of penicillin versus clindamycin in the treatment of odontogenic infections. J Oral Maxillofac Surg. 1988;46:1065-1070.
12. Hersh EV. Adverse drug interactions in dental practice: interactions involving antibiotics. Part II of a series. J Am Dent Assoc. 1999;130:236-251.
13. Goodacre CJ, Spolnik KJ. The prosthodontic management of endodontically treated teeth: a literature review. Part II. Maintaining the apical seal. J Prosthodont. 1995;4:51-53.
14. Kim S. Neurovascular interactions in the dental pulp in health and inflammation. J Endod. 1990;16:48-53.
Dr. Bahcall is assistant professor and chair of the Department of Surgical Sciences and director, Postgraduate Endodontic Program, at the Marquette University School of Dentistry. He is a diplomate of the American Board of Endodontics and a fellow of the International College of Dentists. He can be reached via e-mail at jkbmu@aol.com or call (414) 288-6517.