The increased bacterial burden resulting from poor oral hygiene and periodontal diseases may increase the risk for certain respiratory diseases such as pneumonia and chronic obstructive pulmonary disease (COPD). This article examines the relationship between periodontal diseases and respiratory diseases such as bacterial pneumonia.
PERIODONTAL DISEASES
Periodontal diseases (periodontitis) are chronic, infectious diseases resulting from exposure of the supporting tissues of the teeth (the periodontium) to dental plaque, a complex bacterial biofilm that accumulates on the teeth. Exposure of the periodontium to plaque bacteria causes tissue inflammation. Left untreated, periodontitis is a progressive condition that leads to destruction of periodontal ligament and alveolar bone. The prevalence of periodontitis increases with age, rising from 6% in people aged 25 to 34 years to 41% in those aged 65 or older.1 Although periodontitis is often considered a localized condition, there is increasing evidence linking it to systemic conditions such as bacteremia, cardiovascular disease, preterm birth, diabetes, and respiratory diseases.2
BACTERIAL PNEUMONIA
Pneumonia is an inflammation of the lungs caused by fungal, viral, or bacterial infection. Bacterial pneumonia is the most common and treatable form of the disease. Its symptoms include cough with mucus-like sputum, fever, chills, chest pain, and shortness of breath.
The development of bacterial pneumonia depends on the colonization of the oropharyngeal region by potential respiratory pathogens, aspiration of the pathogens into the lower airway, and failure of defense mechanisms to eliminate the bacteria from the lower airway. Although aspiration of small quantities of oral secretions occurs in healthy individuals, especially during sleep, patients with altered consciousness aspirate oral secretions more frequently and in larger amounts.3 Other conditions predisposing to aspiration include stroke, Parkinson’s disease, alcohol abuse,4 and sedative use.5 Multiple defense mechanisms normally operate within the respiratory tract to eliminate aspirated bacteria from the lower airway, but their effectiveness can be impaired by a variety of conditions and circumstances, including malnutrition, smoking, COPD, diabetes, corticosteroid use, and endotracheal or nasogastric intubation.5
Bacterial pneumonia can be classified as either community-acquired pneumonia (CAP) or hospital-acquired (nosocomial) pneumonia. Up to 5.6 million cases of CAP occur annually in the United States.6 In the outpatient setting, the mortality rate is 5% or less. However, among the 1.1 million CAP cases that require hospitalization, the mortality rate ranges from 12% in the general population to 40% in those admitted to intensive care units (ICUs).6 CAP is caused by aspiration of bacteria that normally reside in the oropharynx, such as Streptococcus pneumoniae, Haemophilus influenzae, and Mycoplasma pneumoniae.7
Pneumonia is the second most common infection in institutional settings, accounting for 10 to 15% of hospital-acquired infections, and has a 20% to 50% mortality rate.4 In contrast to CAP, nosocomial pneumonia is usually caused by bacteria that colonize the oropharynx from the environment,8 including opportunistic pathogens such as Pseudomonas aeruginosa, Staphylococcus aureus, Acinetobacter species, and gram-negative enteric bacteria such as Klebsiella pneumoniae, Escherichia coli, and Enterobacter species.5
OROPHARYNGEAL COLONIZATION BY POTENTIAL RESPIRATORY PATHOGENS AND PNEUMONIA
Oropharyngeal colonization by respiratory pathogens is more common in institutionalized patients who have teeth or dentures than in edentulous patients who do not wear dentures.8 Diminished salivation and lower salivary pH may promote colonization by respiratory pathogens. These conditions can occur in ill patients and among those receiving various medications.9 Colonization by gram-negative bacteria (GNB) that are often the cause of pneumonia is common in elderly patients who are debilitated, hospitalized, or living in a nursing home. In one study, 43% of elderly patients admitted to the hospital had oropharyngeal GNB.10
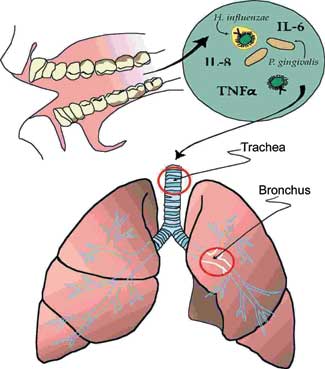 |
| Figure 1. Pathogenic bacteria that can cause respiratory disease colonize the dental plaque biofilm on the teeth. They can then be shed in high numbers into the oral secretions and are in a milieu with cytokines and other biologically active molecules from inflamed gingiva. These secretions may then be aspirated into the lower airway to contribute to infection and inflammation in the lung.(From reference 8, used with permission.) |
Patients who are hospitalized or reside in a nursing home often have more dental plaque accumulation than their community-dwelling counterparts.8 Poor oral hygiene can foster oropharyngeal colonization by bacteria that can be respiratory pathogens (Figure 1). Oral hygiene and colonization rates were evaluated in a study comparing medical ICU patients with age-matched outpatients upon initial presentation to a dental school clinic.9 The mean plaque score was significantly higher in the ICU patients than in the dental subjects (1.9 versus 1.4, respectively, P < 0.005). Colonization of dental plaque and/or oral mucosa by potential respiratory pathogens was found in 65% of the ICU patients but in only 16% of patients in a preventive dentistry clinic (P < .005). The potential respiratory pathogens identified in the ICU patients included S. aureus, P. aeruginosa, and a number of different enteric GNB. Several patients had oropharyngeal colonization by two or more potential pathogens. Respiratory pathogen colonization was correlated with antibiotic use, suggesting that the liberal use of antibiotics in the hospital environment promotes oral colonization by respiratory pathogens (possibly by inhibiting the normal bacterial flora that competes against and prevents colonization by pathogenic bacteria). Possible sources of these bacteria may include other patients, staff members, and contaminated items such as inhalers, mouthrinses, food, and beverages.
| Table 1. Potential pathogens in the dental plaque of ICU patients 11 | ||||||||||
|
The amount of dental plaque in ICU patients typically increases over time.11 In addition, the longer patients remain in the ICU, the more likely their dental plaque may be colonized by potential pathogens (Table 1).11
The association between dental plaque colonization and nosocomial infection was explored in a study of ICU patients.11 Compared with patients who did not have dental plaque colonization on admission or on the fifth day of their ICU stay, those who did were almost 10 times as likely to develop nosocomial pneumonia or bacteremia (P < .001). Dental plaque colonization by respiratory pathogens was found in 4 of the 5 patients who acquired pneumonia while in the ICU. In each of these 4 patients, the same pathogens—either P. aeruginosa or Acinetobacter baumannii—were isolated from both dental plaque and tracheal aspirate. In 2 patients, the pathogen appeared first in dental plaque. In the other 2 patients, the pathogen was identified concurrently in dental plaque and tracheal aspirates. In this study, 78% of patients were mechanically ventilated for more than 2 days.
| Table 2. Risk factors for ventilator-assisted pneumonia (VAP)12 | ||||||||||||||||||
|
In another study, investigators evaluated colonization as a risk factor for ventilator-associated pneumonia (VAP) caused by enteric GNB or Pseudomonadaceae.12 Of the 33 bacterial species identified in bronchoscopic specimens, 97% had previously colonized the oropharynx, 85% had previously colonized the trachea, and 45% had previously colonized the stomach, indicating that the oropharynx and trachea—but not the stomach—are important sources of bacteria that cause VAP. Colonization of the oropharynx or trachea significantly increases the risk of VAP (Table 2).
In mechanically ventilated patients, oropharyngeal colonization by GNB typically occurs within 48 to 72 hours following admission to the ICU.13 Oropharyngeal bacteria enter the lungs in oral secretions that leak past the cuff of the endotracheal tube.14 VAP occurs in 20% to 25% of mechanically ventilated patients and is associated with a mortality rate of 50% to 80%.13
Similar observations have been made in nursing home patients. In a study of long-term care residents and age-matched outpatients, the long-term care group had a higher plaque index (2.3 versus 1.2, P ≤ .001) and a greater amount of denture plaque (1.4 versus 0.3, P ≤ .001) than the outpatient group.15 The degree of dental plaque colonization by respiratory pathogens was significantly different between the groups, with 14% of the long-term care residents having respiratory pathogens comprising ≥1% of the cultivable dental plaque bacteria, while 0% of the outpatients were colonized (P ≤ .05). Within the long-term care group, the colonized patients had a higher mean plaque index (2.6 versus 2.2, P ≤ .05) and a higher incidence of COPD (43% versus 0%, P ≤ .01) than the uncolonized patients. It thus appears that the mouths of nursing home residents are more likely to be colonized by respiratory pathogens than are community-dwelling subjects.
These findings suggest that improved oral hygiene and effective periodontal care could reduce the numbers of pathogenic bacteria in the mouth, therefore preventing the onset of serious respiratory infection in vulnerable subjects. Although oral hygiene measures are a component of nursing care, implementation of such measures is difficult in some patients, such as those who are orally intubated. However, intervention studies show that oral hygiene measures can reduce the incidence of VAP.
| Table 3. Effect of application of chlorhexidine in mechanically ventilated patients16 | ||||||||||
All reductions significant at P < .05 |
The effectiveness of chlorhexidine gluconate was examined in patients placed on mechanical ventilation after cardiac surgery. Patients were randomly assigned to receive either treatment or placebo. For those in the treatment group, 0.12% chlorhexidine gluconate was rigorously applied twice daily to buccal, pharyngeal, gingival, tongue, and tooth surfaces. Patients in both groups also received standard oral care according to the ICU’s protocol. The use of chlorhexidine gluconate significantly reduced the incidence of respiratory infections, the need for systemic antibiotics, and the mortality rate (Table 3).16
Colonization of plaque by respiratory pathogens is also a problem in elderly patients, who often use medications that reduce salivary flow, such as nonsteroidal anti-inflammatory drugs and diuretics. In a study of long-term care residents, pneumonia was diagnosed in 25% of patients with xerostomia but in only 11% of patients with normal salivary flow.17 In the long-term care setting, other known risk factors for pneumonia include impaired function and cognition, use of tracheostomy and feeding tubes, and a history of stroke—factors that can also lead to poor oral hygiene.15
The value of oral care in preventing pneumonia was demonstrated in a 2-year study of elderly, physically handicapped, or mentally impaired nursing home residents.18 Although some patients in the control group brushed their teeth by themselves once daily or irregularly, none received professional dental care. In contrast, patients in the oral care group had toothbrushing performed by a nurse or caregiver after each meal. When toothbrushing was not efficient, 1% povidone iodine was applied to the oropharynx. In addition, all patients in the oral care group received weekly professional care for plaque and calculus control. Compared with the control group, the oral care group had a lower incidence of pneumonia (11% versus 19%, P < .05) and a lower pneumonia-related mortality rate (8% versus 16%, P < .01)
According to a 1995 survey of US nursing homes, 60% of facilities had no regular dental services available to residents.19 Dental care for elderly individuals will become increasingly important as this segment of the population grows. In 2000, 35 million individuals—12% of the total US population—were 65 years or older.20 Projections indicate that elderly persons will number 69 million—20% of the total population—by 2030.21
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
Poor oral hygiene and periodontitis may also be associated with other respiratory diseases such as COPD, which affects up to 15 million people and is the fourth leading cause of death in the United States.6 COPD encompasses emphysema and chronic bronchitis, with signs of chronic bronchitis occurring in more than 80% of patients. Although no study has established that periodontitis causes COPD, the potential association between the two diseases is intriguing. Associations between respiratory diseases and oral health in community-dwelling populations were first determined by analysis of the National Health and Nutrition Examination Survey I data set.22 This database contains information on the general health status of 23,808 individuals. Of these, 365 individuals reported a respiratory condition that was assessed by a study physician. These subjects were categorized as having a confirmed chronic respiratory disease (chronic bronchitis or emphysema) and/or acute respiratory disease (influenza, pneumonia, acute bronchitis), or not having a respiratory disease. Logistic regression analysis revealed that poor oral hygiene and smoking status were statistically associated with chronic respiratory disease. The study of Hayes et al23 found periodontitis, measured as alveolar bone loss assessed from periapical radiographs, to be an independent risk factor for COPD in adult males enrolled in the VA Normative Aging Study.
To verify these results, a study was performed to assess associations between poor oral health and chronic lung disease by analyzing data from the National Health and Nutrition Examination Survey III, which documents the general health and nutritional status of randomly selected US subjects from 1988 to 1994.24 Subjects reporting a history of bronchitis and/or emphysema were together considered as having COPD. Subjects with COPD had on average, more periodontal attachment loss (mean clinical attachment level [CAL]=1.48±1.35; mean±SD) than those without COPD (CAL = 1.17± 1.09). To simultaneously control for multiple variables that may confound statistical analysis, adjustments were made for gender, age, race, education, income, dental treatment history, alcohol consumption, diabetes status, and smoking status. The risk for COPD appeared to be significantly elevated when CAL was found to be severe (≥ 2.0 mm) when compared to the healthy group (< 2.0 mm; odds ratio = 1.35, 95% CI 1.07 to 1.71). Furthermore, the odds ratio was 1.45 (95% CI 1.02 to 2.05) for those who had ≥3.0 mm CAL.
| Table 4. Levels of lung function by dental health status24 | ||
* Significantly greater lung function when compared to those with more mean attachment loss (p < 0.0001, weighted t-test). |
The levels of lung function as related to periodontal status were also considered and are presented in Table 4. A trend was noted in that lung function appeared to diminish as the amount of attachment loss increased. No such trend was apparent when gingival bleeding was considered.
These studies provide preliminary evidence that oral disease such as periodontitis may be associated with COPD. Further epidemiologic and randomized intervention studies must be performed to determine if oral health status plays a causal role in progression of COPD.
BIOLOGICALLY PLAUSIBLE MECHANISMS
Several biologically plausible mechanisms have been put forth to explain how periodontitis can lead to respiratory disease. Salivary enzyme activity is increased in periodontitis and can promote the adhesion of pathogenic bacteria to the oral surfaces, thereby altering oropharyngeal colonization patterns.8,26 In addition, oral bacteria involved in periodontitis can stimulate oral tissues and periodontium to release cytokines, which are proteins involved in cellular interactions and immune responses. These cytokines can promote adhesion of respiratory pathogens to mucosal surfaces, thereby leading to oropharyngeal colonization.
Periodontitis may also affect pathogen adhesion to respiratory epithelium. In vitro studies indicate that the presence of Streptococcus gordonii, a key bacteria in dental plaque formation, enhances the ability of pathogens such as H. influenzae to adhere to respiratory epithelial cells.27 In response to bacterial adhesion, respiratory epithelial cells may release cytokines and attract neutrophils, which in turn release proteolytic enzymes that damage the epithelium and increase its susceptibility to infection.4,24,27 In addition, cytokines released from inflamed periodontal tissues may enter the respiratory tract in aspirated saliva, triggering the same sequence of events, including neutrophil recruitment, epithelial damage, and infection.24
CONCLUSION
Poor oral hygiene leads to an increase in the mass and complexity of dental bacterial plaque. Periodontitis can result and may complicate subsequent efforts to improve oral hygiene. In susceptible patients such as those who are debilitated, hospitalized, or residing in long-term care facilities, this increased bacterial burden may increase the risk of pneumonia and may also play a role in exacerbation or progression of COPD. Improving oral hygiene and treating the periodontal disease could decrease oropharyngeal colonization by pathogenic bacteria and thereby reduce the significant costs, morbidity, and mortality associated with serious respiratory infections in vulnerable patients.
Practitioners can stay current on new research on the periodontal-systemic disease relationships by accessing the American Academy of Periodontology Web site at perio.org.
References
1. Vargas CM, Kramarow EA, Yellowitz JA. The Oral Health of Older Americans. Hyattsville, Md: National Center for Health Statistics; 2001. Aging Trends, No. 3.
2. Scannapieco FA. Position paper of The American Academy of Periodontology: periodontal disease as a potential risk factor for systemic diseases. J Periodontol. 1998;69:841-850.
3. Johanson WGJ. Overview of pneumonia. In: Cecil RL, Bennett JC, Goldman L, eds. Cecil Textbook of Medicine. Philadelphia, Pa: WB Saunders Co; 2000:437-439.
4. Mojon P. Oral health and respiratory infection. J Can Dent Assoc. 2002;68:340-345.
5. Hospital-acquired pneumonia in adults: diagnosis, assessment of severity, initial antimicrobial therapy, and preventive strategies. A consensus statement, American Thoracic Society, November 1995. Am J Respir Crit Care Med. 1996;153:1711-1725.
6. Niederman MS, Mandell LA, Anzueto A, et al, for the American Thoracic Society. Guidelines for the management of adults with community-acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med. 2001;163:1730-1754.
7. Johanson WGJ. Aspiration pneumonia. In: Cecil RL, Bennett JC, Goldman L, eds. Cecil Textbook of Medicine. Philadelphia, Pa: WB Saunders Co; 2000:1616.
8. Scannapieco FA. Role of oral bacteria in respiratory infection. J Periodontol. 1999;70:793-802.
9. Scannapieco FA, Stewart EM, Mylotte JM. Colonization of dental plaque by respiratory pathogens in medical intensive care patients. Crit Care Med. 1992;20:740-745.
10. Preston AJ, Gosney MA, Noon S, et al. Oral flora of elderly patients following acute medical admission. Gerontology. 1999;45:49-52.
11. Fourrier F, Duvivier B, Boutigny J, et al. Colonization of dental plaque: a source of nosocomial infections in intensive care unit patients. Crit Care Med. 1998;26:301-308.
12. Bonten MJ, Bergmans DC, Ambergen AW, et al. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am J Respir Crit Care Med. 1996;154:1339-1346.
13. Treloar DM, Stechmiller JK. Use of a clinical assessment tool for orally intubated patients. Am J Crit Care. 1995;4:355-360.
14. Genuit T, Bochicchio G, Napolitano LM, et al. Prophylactic chlorhexidine oral rinse decreases ventilator-associated pneumonia in surgical ICU patients. Surg Infect (Larchmt). 2001;2:5-18.
15. Russell SL, Boylan RJ, Kaslick RS, et al. Respiratory pathogen colonization of the dental plaque of institutionalized elders. Spec Care Dentist. 1999;19:128-134.
16. DeRiso AJ II, Ladowski JS, Dillon TA, et al. Chlorhexidine gluconate 0.12% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109:1556-1561.
17. Terpenning M, Bretz W, Lopatin D, et al. Bacterial colonization of saliva and plaque in the elderly. Clin Infect Dis. 1993;16(suppl 4):S314-S316.
18. Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. J Am Geriatr Soc. 2002;50:430-433.
19. Gift HC, Cherry-Peppers G, Oldakowski RJ. Oral health care in US nursing homes, 1995. Spec Care Dentist. 1998;18:226-233.
20. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: US Census Bureau; 2002. Census 2000 Special Reports.
21. US Census Bureau. Aging in the United States—Past, Present, and Future. Washington, DC: US Census Bureau. 1997. Wallchart.
22. Scannapieco FA, Papandonatos GD, Dunford RG. Associations between oral conditions and respiratory disease in a national sample survey population. Ann Periodontol. 1998;3:251-256.
23. Hayes C, Sparrow D, Cohen M, et al. The association between alveolar bone loss and pulmonary function: the VA Dental Longitudinal Study. Ann Periodontol. 1998;3:257-261.
24. Scannapieco FA, Ho AW. Potential associations between chronic respiratory disease and periodontal disease: analysis of National Health and Nutrition Examination Survey III. J Periodontol. 2001;72:50-56.
25. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(5 pt 2):S77-S121.
26. Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoc











