Recurrent aphthous ulceration (RAU) also known as aphthous ulceration, recurrent aphthous stomatitis, or in the vernacular as canker sores, is the most common oral ulceration. These ulcers occur several times per year in an otherwise healthy individual. Presently, the cause is unknown although there is reasonable data to suggest an autoimmune etiology.1-5 The occurrence of ulcers is believed to be related to stress, trauma, and hormonal or chemical mediators (hypersensitivity).1 These lesions affect 20% of the population, are painful and appear on the non-keratinized (movable) mucosa including the lips, buccal mucosa, floor of mouth, and soft palate. The lesions are seen more commonly in higher socioeconomic groups, females, children, young adults and non-smokers.1,2 There is also a strong familial propensity.1-5
Three types of RAU are recognized, including minor (Mikuliicz aphthae), major (Sutton’s disease or periadenitis mucosa necrotica recurrens), and herpetiform.4 The minor type, which represents the most common presentation, usually appears as one to four superficial ulcerations that are less than 1 cm in size. They heal in 7 to 10 days without scarring. The major form presents as one or two deep ulcerations that are greater than 1 cm in size. They heal in 2 to 6 weeks with scarring and recur more frequently. In fact, when one ulcer is healing another may appear.5,6 Both major and minor aphthae usually present as circular or oblong target lesions with a white pseudomembraneous center surrounded by erythema. The herpetiform type is the least common, and usually appears in crops of 10 to 50 superficial ulcerations that are approximately 1 to 2 mm in size, and heal in 7 to 10 days without scarring. Individual lesions are very small, and may appear similar to herpes lesions (hence the name herpetiform).2,4
There are many vesicular-ulcerative conditions that may appear in the mouth and can be confused with RAU, including primary herpetic gingivostomatitis, Behcet’s syndrome, cyclic neutropenia, pemphigus vulgaris, benign mucous membrane pemphigoid, herpangina, and Crohn’s disease. These conditions should be considered prior to initiating therapy for RAU. Furthermore, oral ulceration may be indicative of underlying or developing systemic disease, the definitive diagnosis of which may not be possible until later in the disease process. In such cases, it is the dentist’s responsibility to carefully follow proper differential diagnosis protocol in order to determine the most appropriate working diagnosis. Due to the fact that certain systemic diseases may be in the developing stage, the dentist should continually monitor the patient, and collaborate with medical colleagues to determine if a definitive diagnosis becomes evident.
Here a case report is presented that describes the diagnostic process that was followed for a patient presenting with atypical oral ulceration.
Case Report
A 44-year-old caucasian female presented to the Howard University College of Dentistry emergency dental clinic in January of 2000. Her otolaryngologist referred her for an evaluation.
Her chief complaint was the inability to “eat, sleep, or talk for the past 3 weeks.” The history of previous illness indicated that the patient’s oral problems had been ongoing with periods of exacerbation and remission for approximately 10 years. She was previously seen more than 10 years ago at the Georgetown University Medical Center for another oral condition, determined to be herpes zoster. During the time of the examination, the patient was using a dexamethasone elixir (0.5 mg per 5 mL, rinse and expectorate) without relief. A lidocaine rinse was helpful only for brief periods of time. She was also taking 800 mg acyclovir (Zovirax) four/five times a day, but reported no relief with this medication. The patient felt as if she had a fever and reported body aches, but was unsure if this was related to her oral condition or the flu. She also had a prescription for clotrimazole (Mycelex) troches, but she had not taken this medication.
The medical history was positive for asthma, chronic sinusitis, hypertension, previous steroid therapy, and diabetes. The patient used Proventyl (albuterol sulfate), Flovent (fluticasone proprionate), and Seravent (salmeterol xinafoate) for asthma. The patient used Sinutabs (OTC) for sinusitis. Also, she had weekly allergy injections. She reported gestational diabetes. She recently had been evaluated for diabetes, but the results of her hemoglobin A1c were not available. The patient was scheduled for a glucose tolerance test. Her family history noted that her father was 64 years of age with a history of diabetes and hypertension. Her mother was a 61-year-old with a history of hypertension. Her sister was 41 and had a history of hypertension. Her one female child was in good health. At the initial examination, she reported smoking one half pack of cigarettes daily, but did not use alcohol.
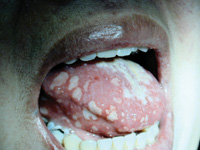 |
| Figure 1. Multiple oral ulcers of the tongue. |
Clinically, lymphadenopathy was not detected. There were multiple intraoral lesions ranging from < 0.25 cm to 1.5 cm in diameter, covering both the dorsal and ventral surfaces of the tongue, buccal mucosa bilaterally, and maxillary vestibular area adjacent to the lips. Many of the lesions coalesced to form larger lesions. The lesions were irregularly shaped, and most presented as ulcers with a pseudomembrane and little or no surrounding erythema. The tongue had a white coating. Pain associated with the lesions prevented the completion of the clinical examination (Figure 1).
The differential diagnoses included viral lesions, candidiasis, and oral vesiculo-bullous disease. The appearance was atypical of oral aphthae due to the absence of erythema surrounding the lesions, and the number of lesions.
In consultation with the patient’s physician, laboratory studies were performed, including a complete blood count, and serum levels of iron, ferritin, serum folate, and vitamin B12. A viral culture and biopsy were also performed. She was encouraged to begin antifungal therapy immediately with clotrimazole 10 mg troches (five times per day for 14 days). A prescription was written for famciclovir 500-mg to be taken one capsule twice a day for 10 days) to begin immediately after the viral culture. The patient was encouraged to use a topical mixture of diphenhydramine (Benadryl) and Maalox mixed in a 1:1 ratio.
 |
 |
| Figure 2a. Low-power hematoxylin and eosin photomicrograph demonstrates inflammation and ulceration. | Figure 2b. Medium-power hematoxylin and eosin photomicrograph demonstrates nonspecific inflammation. |
The biopsy from the dorsum of the tongue demonstrated a nonspecific ulceration with a chronic lymphoplasmacytic response in the connective tissue (Figures 2a and 2b). The viral cultures were negative. The hematological studies were all within normal limits (including the prior A1c and glucose tolerance test). The lesions were diagnosed as herpetiform oral aphthous ulcers. The patient was placed on a descending dose regimen of dexamethasone elixir, and systemic steroids, and the lesions disappeared during the next 3 weeks. Subsequently, oral lesions recurred, usually in association with the patient’s menstrual cycle. The dexamethasone therapy was used successfully for treatment of the recurring episodes. The patient recently began experiencing gastrointestinal symptoms and has been referred to a gastroenterologist to evaluate the possibility of Crohn’s disease.
Discussion
The differential diagnosis in this case included candidiasis, primary herpetic gingivostomatitis, herpes zoster, herpangina, Behcet’s syndrome, cyclic neutropenia, pemphigus vulgaris (PV) or benign mucous membrane pemphigoid (BMMP), Crohn’s disease, and recurrent aphthous ulcers.
Primary herpetic gingivostomatitis is a viral infection that mainly affects children and young adults. The etiology is the Herpes simplex virus-1. Clinical presentation includes fever, malaise, lymphadenopathy, and pain in the oral cavity. Intraorally, vesicular lesions appear, which rupture within 24 hours, leaving painful, small, superficial ulcers. These lesions will appear on the keratinized (bound) and non-keratinized (movable) mucosa. Lesions will heal in 10 to 14 days without scarring. The herpes virus may then reside in a ganglion, periodically activated. The most common result of activation is recurrent herpes labialis. Diagnosis can involve a biopsy to identify viral inclusions. Antiviral medications can be used during the vesicle stage to lessen the duration of the outbreak.6,7
Herpes zoster is a secondary viral infection due to the Varicella virus. Those most often affected are the elderly and immunosuppressed. Clinically, the ulcerations will affect the second and third branches of the trigeminal nerve on one side of the face or oral cavity only, rarely crossing the midline. The skin lesions appear as erythematous papules, then vesicles that rupture and become crusted. The oral lesions appear as superficial ulceration with an erythematous border. The lesions will eventually heal without scarring. Diagnosis is usually made based on the clinical evaluation.6,8
Behcet’s syndrome is a chronic multisystemic inflammatory disease. The etiology is uncertain, and several areas of the body are involved. The mucosal surfaces of the eyes, mouth, and genitals are affected by ulcerations that appear similar to aphthous ulcers. The ulcerations are very similar in size to the minor form of aphthae. Behcet’s syndrome can be associated with arthritis. The ulcerations heal in 7 to 10 days, but recurrence is frequent. Corticosteroids are indicated for treatment. The diagnosis can be made on the basis of the clinical presentation. In contrast to aphthae, Behcet’s ulcerations appear six or more at a time, and have a larger erythematous ragged border. One diagnostic method is a pathergy test. This is a tuberculin-like skin reaction from an injection of an inert substance such as sterile saline solution. This is unique to Behcet’s.6,9
Cyclic neutropenia is manifested by cyclic reduction in the number of circulating neutrophils. The cause is unknown, but an autoimmune etiology has been suggested.6 The reduced number of neutrophils occur routinely at 3-week intervals, and last for 1 to 3 days. The recovery period is 5 to 8 days. The condition is usually seen in infants and young children, but can appear at any age. During episodes of reduced numbers of neutrophils, symptoms include a low-grade fever, malaise, headaches, skin infections, and alveolar bone loss. These oral ulcerations are painful, with a central whitish pseudomembrane and an erythematous border. The ulcerations are generally less than 1 cm in diameter. They can appear anywhere in the oral cavity. The diagnosis is made by following the number of circulating neutrophils over a period of 6 to 8 weeks.6,10
PV and BMMP are autoimmune conditions that affect the skin and mucous membranes of the body. Women are affected more often than men. These conditions may appear at any age, but are seen more commonly in adults. PV is associated with antibodies against the prickle cell layer of the epithelium. BMMP is associated with antibodies against the basement membrane of the epithelium. These two conditions result in blisters that create desquamation in the oral cavity and other mucous membrane sites. They can present in any area of the mouth. Vesicles usually appear first, but are rarely seen clinically. The ulcerations are extremely painful. The size of the lesions is variable. BMMP can result in scarring and blindness, and PV can prove fatal because the patient is never cured of the disease, and long-term steroid treatment results in susceptibility to severe infection.6 The diagnosis is based on a positive Nikolsky sign (pressure to the area results in blister formation) and confirmation with a biopsy and a microscopic presentation consistent with supra-basilar clefting for pemphigus and sub-basilar clefting for pemphigoid.6,11,12 Immunologic diagnosis with direct or indirect immunofluorescence is often helpful.11,12,13
Herpangina is an infection caused by the Coxsackie A virus. Children and young adults are usually affected. This condition presents with sore throat, headache, dysphagia, malaise, and oral ulcerations. In 24 to 48 hours vesicles appear on the soft palate and oropharynx. The vesicles rupture and form small painful superficial ulcers that are present for 7 to 10 days. The ulcerations in the oral cavity are usually limited to the soft palate. Diagnosis is usually based on the clinical presentation, but a biopsy can be used to establish the infection.6,14
Crohn’s Disease (CD) is a chronic inflammatory bowel disease that usually affects the distal portion of the small intestine (ileum). The etiology is unknown, although an autoimmune cause is suspected.6,15 This condition most often affects young women in the third decade of life. Symptoms include abdominal pain, diarrhea, weight loss, low- grade fever, rectal bleeding, arthritis, uveitis, and oral nodules or ulcers. Ten to 20% of CD patients present with oral lesions, and the oral lesions may present before gastrointestinal involvement. Typically, the oral lesions affect the buccal mucosa, mucobuccal fold, vestibules, and ventral tongue. The oral lesions may have a cobblestone appearance. When ulcerative lesions are seen they can have a “snail track”-like appearance, and may be painful (pyostomatitis vegetans). A biopsy of the lesion may reveal non-necrotizing granulomas or markedly edematous acantholytic epithelium with infiltrating eosinophils. In this case, the patient’s oral lesions were suggestive of CD. However, definitive diagnosis of CD is by a gastrointestinal biopsy demonstrating granulomatous inflammation (which was not demonstrated in this patient) and laboratory work-up.6,13,15-17 It is possible that oral lesions may present months or even years before the intestinal signs and symptoms.13,17
This case demonstrates that diagnosis must sometimes rely upon a well-conceived and developed series of laboratory tests and specific therapies to rule out disorders in a differential diagnosis. This particular case posed numerous diagnostic dilemmas. The initial working diagnosis was oral candidiasis and/or an unknown viral infection. A regimen of antifungal medication (Mycelex [clotrimazole] 10-mg troches 5/day for 2 weeks) was used without success, which tended to rule out Candida as a cause. An antiviral medication (famciclovir [Famvir] one 500-mg capsule 2/day for 10 days) was also used without success, and viral cultures were negative, which tended to rule out a viral causation. The complete blood count was normal, which tended to rule out cyclic neutropenia as a cause. The serum analysis ruled out potential contributing factors such as anemia and diabetes. The biopsy demonstrated an appearance consistent with an oral autoimmune condition, but not PV or BMMP. The initial rinse and expectorate steroid regimen was insufficient to treat the patient’s problematic condition. The combined topical and systemic therapy was adequate.
Clinicians unfamiliar with oral autoimmune conditions or the use of corticosteroids may experience treatment failure if an inappropriate regimen is prescribed. An insufficient steroid medication regimen may complicate the diagnostic process by not providing enough medication to influence the disease process.
Through the processes of evaluation and elimination, the present working diagnosis is recurrent aphthous ulcers (herpetiform type), although CD still remains a possibility. The working diagnosis was rendered more difficult because the presentation was atypical for aphthae due to the extent of the condition, the irregular shape of the lesion, and lack of peripheral erythema.
CONCLUSION
There are many pathological conditions that have similar clinical presentations to RAU. The diagnosis of RAU relies primarily upon a well-defined history. Ulcerations tend to be transient and recurring since childhood or early adulthood. In cases when the oral presentation is unusual and the history is vague, a biopsy may be helpful in determining the diagnosis. If the histological diagnosis is not specific, a hematological assessment is advised. The case presented was a diagnostic challenge. The clinical, histological, and hematological evaluation was necessary to rule out a number of conditions.
This case demonstrates that not all lesions detected in the oral cavity (oral mucosa) have a clearly defined etiology. While the diagnosis of recurrent aphthous ulceration of the herpetiform type was made, there is a suggestion that a relationship to CD exists. In this case, regular follow-up and close consultation with the patient’s physician/gastroenterologist is a necessity.
References
1.Regezi JA, Sciubba JJ. Oral Pathology Clinical-Pathological Correlations. Philadelphia, Pa: WB Saunders Company; 1989:46-53.
2.Bhaskar SN. Synopses of Oral Pathology. 6th ed. St Louis, Mo: CV Mosby Company; 1986:443-445.
3.Porter SR, Scully C, Penderson A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 1998;9:306-321.
4.Cooke BED. Recurrent oral ulcers. Br J Dermatol. 1969;81:159-161.
5.Field EA, Brookes V, Tyldesley WR. Recurrent aphthous ulcerations in children: a review. Int J Paediatr Dent. 1992;2:1-10.
6.Neville BW, Damm DD, Allen CM, et al. Oral and Maxillofacial Pathology. Philadelphia, Pa: WB Saunders Company; 1995:181, 188, 192, 239, 423, 424, 559, 563, 567, 620, 621.
7.Cohen SG, Greenberg MS. Chronic oral herpes simplex virus infection in immunocompromised patients. Oral Surg Oral Med Oral Pathol. 1985;59:465-471.
8.Eisenberg E. Intraoral isolated herpes zoster. Oral Surg Oral Med Oral Pathol. 1978;45:214.
9.Mangelsdorf HC, White WL, Jorrizo JL. Behcet’s disease. Report of twenty-five patients from the United States with prominent mucocutaneous involvement. J Am Acad Dermatol. 1996;34:745-750.
10. Dale DC, Hammond WP. Cyclic neutropenia: a clinical review. Blood Review. 1988;2:178-185.
11. Laskaris G Stoufi E. Oral pemphigus vulgaris in a 6-year-old girl. Oral Surg Oral Med Oral Pathol. 1990;69: 609-613.
12. Ahmed AR, Kurgis BS, Rogers A. Cicatricial pemphigoid. J Am Acad Dermatol. 1991;24:987-1001.
13. Millard HD, Mason DK (eds). Perspectives on 1998 3rd World Workshop on Oral Medicine. Ann Arbor, Mich: University Michigan School Of Dentistry; 2000.
14. Cherry JD, Nelson DB. Enterovirus infections: their epidemiology and pathogenesis. Clin Pediatrics (Philadelphia). 1966;5:659-664.
15. Estrin HM, Hughes RW Jr. Oral manifestations in Crohn’s disease: report of a case. Am J Gastroenterol. 1985;80:352-354.
16. Thornhill MH, Zakrzewska JM, Gilkes JJH. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21: 128-133.
17. Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease: an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
Dr. Farquharson is an assistant professor in the Department of Oral Diagnosis and Radiology at Howard University College of Dentistry and Georgetown University Medical Center, Washington, DC.
Dr. Ajagbe is an assistant professor in the Department of Oral and Maxillofacial Pathology at Howard University College of Dentistry and Georgetown University Medical Center, Washington, DC. He is an oral and maxillofacial pathologist.
He has recently been appointed the chairman of the African American Health Initiative-Oral Health Coalition and is a member of the AAHI executive board. He is also the current dental director of the National Minority AIDS Education and Training center funded by the Health Resources and Services Administration (HRSA).
Dr. Brown is associate professor in the Departments of Oral and Maxillofacial Pathology and Oral Diagnosis and Radiology at Howard University College of Dentistry, and a clinical associate professor in the Department of Otolaryngology at Georgetown University Medical Center. He is past president of the American Academy of Oral Medicine. He can be reached at rbrown@howard.edu.