I just changed the sealer I use in practice after successfully using Kerr Pulp Canal Sealer (KPCS) for 40 years. For an endodontist, this is a big deal.
Ask any endodontist, “What part of your RCT procedure are you least willing to change?” Nine out of 10 endodontists (and the tenth just doesn’t know any better) will say they are most anxious about changing their filling materials because that is the greatest long-term risk to an endodontic specialty practice. Think about it: If you change filling materials because somebody convinced you that a new sealer is better because of blah, blah, blah, and if you are a typical endodontist who does 800 to 1,000 cases a year and the new material starts failing after 3 years, you could have thousands of cases coming back to haunt you! Practices have died over less.
Does this sound like an overdramatization? It isn’t. The last new obturation material to flame out was Resilon, a polycaprolactone-based endodontic composite filling material. Designed by PENTRON to replace traditional gutta-percha and sealers, this insidious material began failing after 6 years, doubling the failures in the example above because, over the additional 3 years, 3,000 more cases were treated with it before the chickens came home to roost. Let’s be conservative and say the fees averaged $500/RCT. That $500 × 3,000 potential failures = $1,500,000 of liability! And that one is not a made-up number.
So, what would convince me to change from the sealer that I have used successfully with my Continuous Wave (CW) Obturation Technique for 40 years? Am I throwing Rickert’s sealer (KPCS)—formulated more than a century ago—over for a new trophy sealer? Absolutely not!
I changed to BC HiFlow Sealer (Brasseler USA) because all of Brasseler’s claims have been proven by our best researchers for more than a decade. The material is completely biocompatible,1-14 and this sealer is also an excellent pulp capping agent that incites odontoblastic proliferation, mineralization, and osteogenesis.15-21 Somehow, at the same time, it has significant antibacterial properties.22-24 All of this is found in a material that seals like MTA, but doesn’t stain dentin.25-33
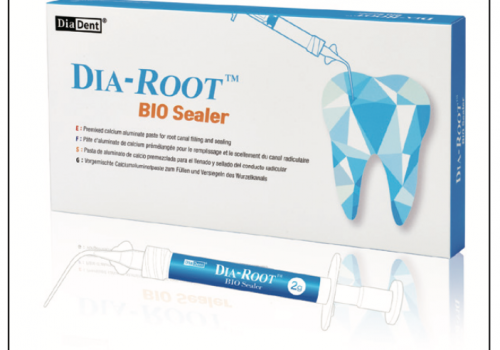
I changed to this sealer because they reformulated BC Sealer to meet my warm gutta-percha obturation needs. The new BC HiFlow Sealer (Figure 1) was designed for higher heat resistance (up to 428°F), and it also has 20% greater radio-opacity. I also changed to this sealer because the functional characteristics of these bio-ceramic materials profoundly change everything in endo obturation—in more ways than first meet the eye.
 |
| Figure 1. Brasseler USA’s BC HiFlow Sealer, designed for warm gutta-percha obturation techniques. This sealer is chemically identical to BC Sealer; the differences are heat resistance up to 428°F and an additional 20% radiopacity. |
This Changes Everything
The literature tells us that BC Sealer has a slight (0.2%) net expansion when it sets34 instead of the significant shrinkage (up to 21%AH+) seen previously in all sealers.35 How does this change things in profound ways? I love this sealer as a practitioner because it streamlines CW Obturation,36 increasing my practice productivity, as well as an educator because it simplifies the technique, making warm gutta-percha obturation more accessible to dentists of all skill and experience levels.
The most complex parts of traditional warm gutta-percha condensation methods have always been:
1. the need to downpack within 4.0 to 6.0 mm of the terminus, even when obturating small, curved molar canals
2. and syringe backfilling these narrow spaces without leaving voids.
CW Obturation—with its dead-soft stainless steel electric heat pluggers (elementsfree [Kerr Endodontics]) and its NiTi hand pluggers (Buchanan Pluggers [Kerr Endodontics]), enabling clinicians to downpack within 4.0 to 6.0 mm of the end of almost any small, curved canal—is a huge improvement over the Schilder technique with its rigid pluggers. However, the CW technique, as done with traditional sealers, requires pre-fitting the electric heat pluggers in the canals before cementing the master cone in the canal with sealer. This pre-fitting routine is done by pressing the appropriate-size plugger into the canal as it is rocked back and forth. This rocking action causes the plugger to work its way into the canal; in the process, the canal accurately bends them to match its curvature (Figure 2a). It was this improvement that enabled a much deeper downpack than the Schilder Warm Vertical Technique while filling all lateral canals in less than 2 seconds (Figures 2b and 2c).
Why have we felt compelled to downpack that far into the canal? Sealers are a necessary component of a successful RCT fill, as GP is not an effective sealing medium; however, all conventional sealers shrink as they set. Because of this sealer shrinkage, our best procedural workaround to prevent this shrinkage from pulling the sealer off canal walls has been to downpack deeply into even small curved canals to thermoplastically move the heat-softened GP into the intaglio of the canal, thereby thinning the sealer layer.
This is a well thought-out procedure, considering previous sealer constraints. It is also set up to backfill voids. Despite voids being clinically the least important part of the CW procedure, seeing a backfill void on a post-RCT radiograph leaves the clinician with disappointment instead of the thrill-of-the-fill.
 |
| Figure 2a. A Continuous Wave (CW) electric heat plugger being fit prior to cementation of gutta-percha and downpacking. Figure 2b. The CW Plugger at the apical extent of the downpack. Figure 2c. A backfill gap between an apical mass of gutta-percha at the canal diameter of 0.4 mm and the 0.65-mm diameter of a 23-ga backfill cannula. |
Good Riddance to Lateral Condensation
Change 21% shrinkage to 0.2% expansion, and, suddenly, nobody cares how thick the sealer layer is; we only care if we can move it into all the lateral irregularities that have been cleaned out. Combine net expansion on setting with extremely low surface tension and high wettability,20 and, suddenly, we find that just a 3.0- to 4.0-mm downpack will fill every nook and cranny in the most complicated anatomy. This pivot in the CW Obturation procedure simplifies the downpack, as electric heat pluggers no longer need to be pre-fit and bent before cementing master cones. This reduced need to achieve depth in the downpack also means it is much easier to backfill without voids.
An easy way to explain the importance of the wetting characteristics of BC HiFlow Sealer over traditional sealers is to consider the difference in technique sensitivity between flowable and traditional composite materials. This surprising ability of BC Sealer to flow into lateral spaces with very little pressure needed has significantly changed the dynamic around cold gutta-percha filling techniques in general practices. Dr. Herb Schilder was known to say, “Lateral condensation of cold gutta-percha is single-cone technique with a conscience.” Now we can say that single-cone obturation with BC Sealer is far better than lateral condensation because (a) this sealer will fill the primary canal next to the master cone and lateral canals 1 to 2 mm long by simply cementing the master cone into a BC Sealer-laden canal (Figure 3); and (b) lateral condensation of cold GP requires over-cutting coronal canal shapes so a spreader (basically a thin wedge) can be forced into the canal (an enormous root-splitting force) to push the master cone aside and allow an inconsequential accessory cone to be placed in that space.
For these reasons, lateral condensation of cold GP—a technique that requires the weakening of teeth to allow a filling method that doesn’t improve the result—is dead and gone. It is a technique without rationale.
 |
| Figure 3. A TrueTooth Replica of a maxillary central incisor filled with single-cone technique and BC Sealer. Note the buccal and lingual fins, the mid-root lateral canal, and the apical accessory canal were all filled due to the extremely low surface tension of BC HiFlow Sealer. With this in mind, there is no longer any credible rationale for the lateral condensation, an obturation technique that requires overcutting coronal shapes to create intra-canalar space for inconsequential accessory points. |
Continuous Wave Obturation 2.0
If BC Sealer fills lateral canals 1.0 to 2.0 mm in length when doing single-cone obturation, why do we need to heat GP up and downpack at all? Unfortunately, lateral canal spaces in molars are way bigger than that. Forget about the 4.0-mm-wide isthmus forms found in mesial roots of lower molars. Forget about the fins, webs, loops, and lateral canals that commonly project off of single primary canals. Be worried about MB2 and MB3 canals in upper molars that bifurcate mid-root off the MB1, turn 90°, and bifurcate before exiting (Figure 4). These can be 7.0 to 8.0 mm in length, so I am still a warm GP guy. With BC HiFlow Sealer, I just don’t have to work as hard to get the 3-D results I expect to see on post-obturation radiographs.
 |
| Figure 4a. A mesial CBCT view of an MB root of a maxillary molar after a fruitless search for the MB2 canal. Note the 4-mm MB2 canal that bifurcates mid-root off the MB1 canal, makes a 90° turn, and exits. Cementing a single GP cone with BC Sealer without a downpack won’t fill this anatomy. Figure 4b. A postoperative CBCT scan, showing the MB2 filled with a shortened CW downpack. |
How does this simplify warm gutta-percha obturation? Primarily by shortening the required downpack distance into the canal. As mentioned above, with the no net-shrinkage of bio-ceramic sealers, the warmed gutta-percha and the sealer beneath it need just half the previous depth of CW downpack to move sealer into the full apical and lateral extents of root canal systems (Figure 5). The shortening of the downpack means that pre-fitting pluggers before cementation of master cones is of little or no importance, and it also means that backfilling can be done with a small, single squirt of GP from a backfill syringe with a sealer-coated backfill cone (my favorite backfilling method), or the gutta-percha the downpack moved to the periphery of the canal can be reheated and condensed back into the canal.
BC HiFlow Sealer also works well for carrier-based obturation. For example, 3.0 mm of sealer is syringed into each canal, an XP-Finisher (Brasseler USA) is used to spread a thin coat of sealer on canal walls, and then the oven-heated obturator is placed 1.0 mm short of full length. With the heat resistance of HiFlow, carrier placement with bio-ceramic sealer is identical to the placement of carriers with traditional sealers, except patients have no postoperative discomfort due to BC HiFlow Sealer’s complete biocompatibility (Figure 6).
 |
 |
| Figure 5a. Master gutta-percha points, cemented in canals with BC HiFlow Sealer. Note the short apical lateral canals filled just by cementing a single GP cone in the palatal root; also note the incomplete lateral fill of the MB root complex. | Figure 5b. CW electric heat pluggers in their final positions after modified CW downpacks to mid-root. Note the MB2 and MB3 complexities filled by bio-ceramic sealer after a short hydraulic wave of condensation. |
 |
| Figure 6. A mandibular molar with severe, multiplanar curvatures of all canals. The D canal was instrumented with a single XP-3D Shaper (Brasseler USA); the mesial canals were shaped with a 15-.06 rotary file. All the canals were filled with BC HiFlow Sealer and EdgeCore gutta-percha carriers (EdgeEndo). |
 |
| Figure 7. A mandibular premolar with extensive internal resorption. This case was treated in a single visit using GentleWave (Sonendo) multisonic cleaning and BC HiFlow bioceramic sealer. Note the wild resorptive pattern filled by a short CW downpack. |
Conclusion
Changing sealers is a big, scary deal for an endodontist because thousands of patients could be hurt if the new sealer fails before a couple of decades go by. BC HiFlow Sealer checks all the required safety boxes, such as biocompatibility, antibacterial, etc; however, the greatest advantage of this sealer (besides its 10-year history of success) is its net-expansion upon setting (Figure 7). Why? Because it is the death knell for lateral condensation. Good riddance. The king is dead, long live the king.
References
- Zhang W, Li Z, Peng B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int Endod J. 2010;43:769-774.
- Ma J, Shen Y, Stojicic S, et al. Biocompatibility of two novel root repair materials. J Endod. 2011;37:793-798.
- Alanezi AZ, Jiang J, Safavi KE, et al. Cytotoxicity evaluation of EndoSequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e122-e125.
- Ruparel NB, Ruparel SB, Chen PB, et al. Direct effect of endodontic sealers on trigeminal neuronal activity. J Endod. 2014;40:683-687.
- Chang SW, Lee SY, Kang SK, et al. In vitro biocompatibility, inflammatory response, and osteogenic potential of 4 root canal sealers: Sealapex, Sankin apatite root sealer, MTA Fillapex, and iRoot SP root canal sealer. J Endod. 2014;40:1642-1648.
- Ciasca M, Aminoshariae A, Jin G, et al. A comparison of the cytotoxicity and proinflammatory cytokine production of EndoSequence root repair material and ProRoot mineral trioxide aggregate in human osteoblast cell culture using reverse-transcriptase polymerase chain reaction. J Endod. 2012;38:486-489.
- Hirschman WR, Wheater MA, Bringas JS, et al. Cytotoxicity comparison of three current direct pulp-capping agents with a new bioceramic root repair putty. J Endod. 2012;38:385-388.
- Zhou HM, Du TF, Shen Y, et al. In vitro cytotoxicity of calcium silicate-containing endodontic sealers. J Endod. 2015;41:56-61.
- Shi S, Bao ZF, Liu Y, et al. Comparison of in vivo dental pulp responses to capping with iRoot BP Plus and mineral trioxide aggregate. Int Endod J. 2016;49:154-160.
- Öncel Torun Z, Torun D, Demirkaya K, et al. Effects of iRoot BP and white mineral trioxide aggregate on cell viability and the expression of genes associated with mineralization. Int Endod J. 2015;48:986-993.
- Liu S, Wang S, Dong Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J Endod. 2015;41:652-657.
- Shinbori N, Grama AM, Patel Y, et al. Clinical outcome of endodontic microsurgery that uses EndoSequence BC root repair material as the root-end filling material. J Endod. 2015;41:607-612.
- Chen I, Karabucak B, Wang C, et al. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod. 2015;41:389-399.
- Khalil WA, Abunasef SK. Can mineral trioxide aggregate and nano-particulate EndoSequence root repair material produce injurious effects to rat subcutaneous tissues? J Endod. 2015;41:1151-1156.
- Zhang S, Yang X, Fan M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int Endod J. 2013;46:923-929.
- Zhang W, Li Z, Peng B. Effects of iRootSP on mineralization-related genes expression in MG63 cells. J Endod. 2010;36:1978-1982.
- Jiang Y, Zheng Q, Zhou X, et al. A comparative study on root canal repair materials: a cytocompatibility assessment in L929 and MG63 cells. ScientificWorldJournal. 2014;2014:463826.
- Machado J, Johnson JD, Paranjpe A. The effects of EndoSequence root repair material on differentiation of dental pulp cells. J Endod. 2016;42:101-105.
- Chen I, Salhab I, Setzer FC, et al. A new calcium silicate-based bioceramic material promotes human osteo- and odontogenic stem cell proliferation and survival via the extracellular signal-regulated kinase signaling pathway. J Endod. 2016;42:480-486.
- Zhang H, Shen Y, Ruse ND, et al. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J Endod. 2009;35:1051-1055.
- Lovato KF, Sedgley CM. Antibacterial activity of EndoSequence root repair material and ProRoot MTA against clinical isolates of Enterococcus faecalis. J Endod. 2011;37:1542-1546.
- Wang Z, Shen Y, Haapasalo M. Dentin extends the antibacterial effect of endodontic sealers against Enterococcus faecalis biofilms. J Endod. 2014;40:505-508.
- Zhang W, Li Z, Peng B. Assessment of a new root canal sealer’s apical sealing ability. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e79-e82.
- Nagas E, Uyanik MO, Eymirli A, et al. Dentin moisture conditions affect the adhesion of root canal sealers. J Endod. 2012;38:240-244.
- Leal F, De-Deus G, Brandão C, et al. Similar sealability between bioceramic putty ready-to-use repair cement and white MTA. Braz Dent J. 2013;24:362-366.
- Ersahan S, Aydin C. Dislocation resistance of iRootSP, a calcium silicate-based sealer, from radicular dentine. J Endod. 2010;36:2000-2002.
- Ghoneim AG, Lutfy RA, Sabet NE, et al. Resistance to fracture of roots obturated with novel canal-filling systems. J Endod. 2011;37:1590-1592.
- DeLong C, He J, Woodmansey KF. The effect of obturation technique on the push-out bond strength of calcium silicate sealers. J Endod. 2015;41:385-388.
- Topçuoğlu HS, Tuncay Ö, Karataş E, et al. In vitro fracture resistance of roots obturated with epoxy resin-based, mineral trioxide aggregate-based, and bioceramic root canal sealers. J Endod. 2013;39:1630-1633.
- Keskin C, Demiryurek EO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod. 2015;41:409-411.
- Kohli MR, Yamaguchi M, Setzer FC, et al. Spectrophotometric analysis of coronal tooth discoloration induced by various bioceramic cements and other endodontic materials. J Endod. 2015;41:1862-1866.
- Shokouhinejad N, Nekoofar MH, Pirmoazen S, et al. Evaluation and comparison of occurrence of tooth discoloration after the application of various calcium silicate-based cements: an ex vivo study.
J Endod. 2016;42:140-144. - Marconyak LJ Jr, Kirkpatrick TC, Roberts HW, et al. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J Endod. 2016;42:470-473.
- Richardson IG. The calcium silicate hydrates. Cem Concr Res. 2008;38:137-158.
- Rezai S, Sobhani H. Measurement and Comparison of Volumetric Shrinkage of Various Sealers Registered by a Video Imaging Technique Device [master’s thesis]. http://www.diva-portal.org/smash/get/diva2:1047923/FULLTEXT01.pdf. Accessed September 8, 2018.
- Continuous wave of obturation. In: Ingle JI, Bakland LK. Endodontics. 5th ed. London, England: BC Decker; 2002:622-623.
Dr. Buchanan owns a private practice limited to endodontics and implant surgery in Santa Barbara, Calif. He is the founder of Dental Education Laboratories, a hands-on training center serving general dentists and endodontists by upgrading their skills with new endodontic and implant technologies. Dr. Buchanan is a Diplomate of the American Board of Endodontics and an assistant clinical professor for the postgraduate endodontic programs at the University of Southern California and the University of California, Los Angeles. He can be reached via the website delendo.com or via email at info@endobuchanan.com.
Disclosure: Dr. Buchanan is a speaker for Brasseler USA and a consultant for Sonendo. He is president of Dental Education Laboratories and Dental Engineering Laboratories. He also has financial interests in Dentsply Sirona, Obtura Spartan, and Sybron Kerr.
Related Articles
Inflection Points in Dental Imaging
Should General Practitioners Place Implants?
Endodontic Shaping Procedures: The Past, Present, and Near Future