The treatment of avulsed teeth has changed rapidly in the last several years. The previously reported poor success rates of reimplanted avulsed teeth (4% to 50%) can be improved to over 90% with a modified approach to treatment.1
Until recently, all avulsed teeth were treated in the same way, which involved reimplantation of the tooth immediately or as soon as possible after the avulsion. This approach was followed regardless of how much time had passed since the avulsion accident and was attempted even if the avulsed tooth was dehydrated and the periodontal ligament (PDL) cells were no longer viable. This philosophy became pervasive, and practitioners did not recommend any other treatment. This occurred despite evidence in the literature that did not support this approach.2-4
Each patient who has suffered an avulsed tooth arrives at the dentist’s office with a specific set of circumstances. The history is evaluated, a diagnosis is made, then treatment is instituted. The treatment of avulsed teeth should, therefore, be dependent upon the specific clinical conditions that present to the dentist. These clinical factors are the following: the physiologic status of the PDL, the stage of root development, and the length of time since avulsion (extraoral time). The availability and use of a specialized storage and preservation medium, a cushioning and retrieval system, topical antibiotics, and enamel matrix protein promoters can increase reimplantation success.1
BIOLOGIC RATIONALE FOR TREATMENT RECOMMENDATIONS
Status of the Development of the Root Apex
In certain instances, it is possible for the pulp tissue in immature avulsed teeth to completely revascularize.4 Researchers differ as to which factor is most influential regarding the incidence of revascularization. Important parameters include the width of the apical foramen, duration of time the tooth is outside the socket, and storage conditions. However, there is agreement that bacterial contamination can prevent revascularization of the pulp. Soaking avulsed teeth in a 1-mg/20-mL solution of doxycycline for 5 minutes prior to reimplantation has been shown to decrease root resorption and—of greater significance—to increase the frequency of pulpal revascularization.4
Physiologic Status of the PDL Cells
The status of PDL cells remaining on the root surface of an avulsed tooth determines the development of replacement root resorption.8 If the PDL cells are healthy, a new attachment to the surrounding alveolar bone is possible. If the PDL cells are metabolically stressed or damaged physically, the root cementum will experience necrosis. A new PDL will not form, and the surrounding alveolar bone will view the root surface as foreign and resorption will occur.9-11 In order for optimum physiologic conditions to be maintained, the PDL cells on the avulsed tooth require nutrients and the proper pH.1,12 In order for proper structure and physiology to be maintained, the cells must be in an appropriate osmotic environment and must be protected from physical damage.13,14
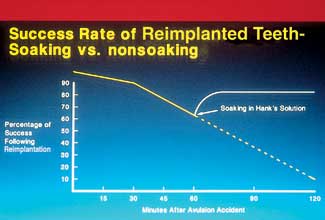 |
| Figure 1. The beneficial effect of soaking vs nonsoaking of avulsed teeth in Hank’s Balanced Salt Solution. |
Many studies have demonstrated the damaging effects that storage can have on PDL cells. It is clear that dry storage, storage in water, and even storage in saliva are damaging to these cells. Water and saliva have a low osmolality and cannot replace lost cell nutrients.13 Even milk, which has received much attention as a storage medium, does not preserve PDL cells well.1 A fluid that has been shown to have the necessary characteristics for preserving cell viability is Hank’s Balanced Salt Solution (HBSS), which is a cell culture medium. HBSS is a pH-balanced cell preserving fluid, not a salt-water solution. It maintains the ideal osmotic pressure for PDL cells and replaces depleted cell metabolites.2 Its use has been shown to maintain cell vitality, reconstitute cells that would otherwise die, and prevent or reduce resorption2,6,7 (Figure 1).
Length of Time the Tooth Is Outside the Socket
The focus of research concerning the treatment of avulsed teeth has been on the length of time elapsed from avulsion to reimplantation. If the avulsed teeth are stored in a supportive medium such as HBSS, the length of time out of the socket is less important then physiologic status of the PDL cells.15,16 Studies have indicated that storage for 4 days in dogs and 2 weeks in monkeys results in <30% resorption following reimplantation.17-19
CLINICAL TREATMENT OF AVULSED TEETH
The treatment of avulsed teeth can be divided into 3 stages: pre-reimplantation, reimplantation, and post-reimplantation. Successful long-term retention of reimplanted avulsed teeth involves a wide range of factors, including treatment directed toward the PDL cells (ie, use of a biologic storage medium and maintenance of PDL cell vitality), timing of pulp extirpation, and splinting of the involved tooth or teeth. Every step in this process is important, and ultimate success is dependent to some degree on all 3 stages. Nevertheless, the pre-reimplantation stage is of primary importance.
Pre-reimplantation Stage
| Table 1. Categories of Avulsed Teeth1 |
| Category 1. Mature apex, < 15 minutes out of mouth. Category 2. Mature apex, 15 minutes to 6 hours in physiologic solution. Category 3. Mature apex, 15 minutes to 1 hour in nonphysiologic solution. Category 4. Mature apex, > 1 hour dry storage. Category 5. Immature apex, < 15 minutes out of mouth. Category 6. Immature apex, 15 minutes to 6 hours in physiologic solution. Category 7. Immature apex, 15 minutes to 1 hour in nonphysiologic solution. Category 8. Immature apex, > 1 hour dry storage. |
| Table 2. Treatment for Each Category of Avulsed Teeth1 |
| Category 1. Mature apex, < 15 minutes out of mouth. (1) Rinse tooth with physiologic solution to remove debris from root surface. (2) Flush socket with sterile water or saline. (3) Reimplant tooth in the socket. Category 2. Mature apex, 15 minutes to 6 hours in physiologic solution. Category 3. Mature apex, 15 minutes to 1 hour in nonphysiologic storage. Category 4. Mature apex, > 1 hour dry storage. Category 5. Immature apex, < 15 minutes out of mouth. Category 6. Immature apex, 15 minutes to 6 hours in physiologic solution. Category 7. Immature apex, 15 minutes to 1 hour in nonphysiologic solution. Category 8. Immature apex, > 1 hour dry storage. |
Patients with avulsed teeth can be categorized into one of 8 categories (Tables 1 and 2). Only patients with avulsed teeth in category 1 benefit from immediate reimplantation. Patients in the other 7 categories benefit from prereimplantation conditioning of affected teeth.
The clinician reimplanting the tooth must be concerned with maintaining the PDL cells in their original state or returning the cells to as healthy a condition as possible. By so doing, the remaining PDL cells will be able to differentiate and reestablish a new PDL. The PDL cells that remain on the root following the avulsion do not have a blood supply and begin to deplete their stored metabolites. In order to maintain optimal cell metabolism, the depleted metabolites must be replaced within 1 hour after avulsion.1 After this time, the untreated PDL cells will undergo necrosis, the cementum will be lost, and root resorption will occur following reimplantation.
Since teeth are rarely reimplanted within this time frame, biologic storage of the avulsed teeth and protection of the PDL cells from physical damage are of paramount importance.20 Many methods of storage have been proposed.6,21 Except for the pH-balanced cell culture media, all of these methods either damage the PDL cells (water and saliva22), or at best are of limited benefit (milk). Milk has been shown to be a compatible short-term storage medium if the avulsed teeth are placed in milk within 15 to 20 minutes following avulsion.4 However, milk only prevents cell death; it does not restore normal cell morphology or the ability of these cells to differentiate and undergo mitosis.23 More significantly, the studies of milk as a storage medium have been performed under ideal conditions.4 In most of these studies, teeth were extracted, immediately placed in milk, and then left for variable periods of time.4 The conclusions regarding milk as a storage medium were thus based on conditions that did not mimic actual clinical conditions.
Teeth that have been outside the mouth for 15 minutes or more should not be immediately reimplanted but rather soaked in a pH-balanced cell-reconstituting medium such as HBSS for 30 minutes and then reimplanted.1,2 Once the teeth have been placed in HBSS, they can remain in that solution for up to 24 hours.1 Some studies have suggested that 70% of PDL cells can remain viable for as long as 4 days in HBSS.4
Even if avulsed teeth are stored in saline or milk from the moment of the avulsion accident, the teeth should still be soaked in HBSS for 30 minutes prior to reimplantation. PDL cells that are stored in milk remain vital but lack the capacity for mitosis and reformation of the PDL.
 |
| Figure 2. Save-A-Tooth System. |
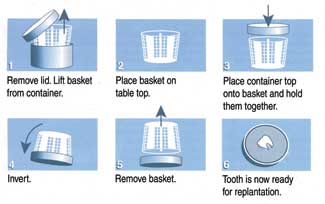 |
| Figure 3. Use of the Save-A-Tooth System. |
 |
| Figure 4. Avulsed tooth on soft sponge. |
Successful reimplantation of avulsed teeth can be achieved if the teeth are stored in an optimal storage environment (OSE) such as the Save-A-Tooth system20 (Figures 2, 3, 4). Save-A-Tooth is a 6-part system that was designed to prevent damage to the PDL cells of avulsed teeth. Each part of the system addresses a potentially damaging situation that can occur between the time of avulsion and reimplantation. Among these situations are (1) removal of debris from the root surface, (2) spillage of storage medium, and (3) removal of the avulsed tooth from the storage container. All of these situations can lead to PDL damage and thus to increased root resorption following reimplantation. The 6 parts of the Save-A-Tooth system and their functions are the following:
(1) Shatterproof container to prevent leakage of HBSS.
(2) Tightly fitting top to prevent spillage during transport.
(3) Removable basket that permits atraumatic removal of teeth.
(4) Suspension net with divider fins that permits atraumatic washing of debris from the tooth surface and prevents bumping of the avulsed tooth against the walls of the container or against another tooth.
(5) Sponge on underside of lid that allows for atraumatic removal of the avulsed tooth.
(6) HBSS, which provides optimum osmotic pressure and replacement of PDL cell metabolites.
There is space on the Save-A-Tooth container to write the patient’s name. One suggested use of this system is in ambulances. If the accident victim has severe injuries and several teeth avulsed, emergency personnel are often reluctant to take the time to find the avulsed teeth and reimplant them. If the Save-A-Tooth system is utilized, a friend or family member can gather the teeth and place them in the container and bring it to the hospital, where the teeth can be reimplanted when the patient is stabilized.
There is little difference in clinical resorption rates of teeth reimplanted within 15 minutes and those reimplanted after many hours of storage in a Save-A-Tooth system, with less than 9% of the teeth stored in the system demonstrating moderate or severe root resorption.1,20 The use of a Save-A-Tooth system is now considered the standard of care for avulsed teeth and is recommended for use by dentists, in ambulances, in emergency rooms, in schools, and at home.1,18
Avulsed teeth with immature roots present a different clinical problem. These teeth are subject to root resorption but also possess the potential for pulpal revascularization.24 The width of the apical foramen is not the most important factor, but rather the prereimplantation conditioning procedures. Teeth with an open apex must be treated differently than teeth with a closed apex.1,18 Avulsed teeth with an open apex should be soaked for 5 minutes in a 1-mg/20-mL doxycycline solution to increase the chance of pulpal revascularization.1,18 Complete revascularization appears to be dependent on the absence of bacteria in the pulpal lumen.4
Therefore, when treating avulsed immature teeth, one must consider the physiologic status of the PDL cells and the potential for pulpal revascularization. If an avulsed immature tooth has been outside the mouth for 15 minutes or less, it should not be reimplanted immediately but should be soaked in the doxycycline solution for 5 minutes, then reimplanted.1,18 In each of these situations, the teeth should be monitored every month for signs of pulpal necrosis and root resorption. If there are signs of resorption, the pulp should be immediately extirpated and apexification procedures instituted.
As an alternate approach for teeth that have been outside the mouth for more than 1 hour in a nonphysiologic medium, a regenerative therapy can be used.18 Since the PDL cells will be necrotic, they are scraped from the root surface and the tooth placed in a sodium hypochlorite solution to remove cellular debris. The root surface is then treated with Emdogain (Biora), an enamel matrix protein solution.18 Emdogain should also be placed in the socket prior to reimplantation. Emdogain has been shown to stimulate the formation of new cementum on root surfaces.25,26
TRANSPORTATION AND CLINICAL MANAGEMENT OF AVULSED TEETH
The means by which an avulsed tooth is transported to the dentist and is then removed from the transportation device can affect the success of reimplantation.1 Consensus recommends against touching the root surface of the avulsed tooth. This recommendation can be difficult to follow. Transport devices such as a paper tissue, handkerchief, and cotton gauze should not be used. If the tooth is transported in a glass or plastic container, the container should have a tightly fitting top so that the contents do not spill.
Retrieving an avulsed tooth from a transportation container without damaging or traumatizing the root can be difficult. The tooth may be covered in debris, which can cause the transport media to be opaque. If it is difficult to see the tooth, reaching for it blindly with a forceps will cause crushing damage to PDL cells. If the container is tipped to remove the liquid, the avulsed tooth can inadvertently fall to the counter top or floor. If the liquid is suctioned, there may not be a medium in which to place the tooth should a delay occur in reimplantation (eg, modifying the socket). The Save-A-Tooth system has a removable basket that permits atraumatic retrieval of the avulsed tooth while also retaining the storage medium if it is needed later.
Crushing damage to the PDL cells of an avulsed tooth has been shown to cause severe root resorption following reimplantation.27 If a dentist is called by a patient or parent regarding transportation of an avulsed tooth, the patient should be told to place the tooth in a Save-a-Tooth, or if not available, a jar with a lid filled with cold, fresh, whole milk, and to try to avoid dry storage.
Reimplantation Stage
Prior to reimplantation, a radiograph is taken to rule out the presence of a root tip or foreign body in the socket. The presence of an alveolar fracture can also be identified. Local anesthesia is administered, and the socket is gently irrigated with sterile water, saline, or an anesthetic solution. If a clot has formed in the socket, it can be gently removed by curettage. The previously treated avulsed tooth is grasped by the clinical crown and inserted into the socket. Steady, gentle pressure should be exerted in an apical direction. It may require as long as 30 to 60 seconds to completely seat the tooth. If the tooth will not seat completely, remove it, place it in a Save-A-Tooth container, and once again curette and irrigate the socket. Surgical removal of the tooth apex is not indicated.
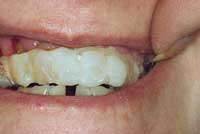 |
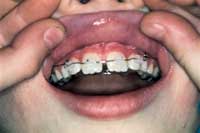 |
| Figure 5. Improper splint. | Figure 6. Physiologic splint. |
When the tooth is in place, it must be stabilized. There are a variety of approaches that can be used, but splinting should place the tooth in physiological function (ie, the splint should hold the tooth firmly in position while allowing slight movement; Figures 5 and 6). The occlusion should be carefully checked prior to finalizing the splint. The ideal splint utilizes orthodontic brackets and a 0.06 wire on the avulsed tooth and one tooth mesial and distal to it.
If orthodontic brackets cannot be placed, GC Fuji Ortho (GC Corporation), a resin reinforced glass ionomer, placed on the facial surface of the teeth is a good substitute. Splints involving arch bars and nylon fishing line or splints that require a channel to be cut in the teeth should be avoided. After the splint is completed, the occlusion should be checked to confirm that excessive force is not being exerted on the reimplanted tooth. In addition, a radiograph with the avulsed tooth splinted into position should be taken to ensure proper placement. Endodontic treatment should not be instituted at this time. The patient is reappointed for the following week. The patient should be prescribed a systemic antibiotic such as Augmentin 250 mg qid or doxycycline 100 mg bid for 7 to 10 days.18 A chlorhexidine rinse is used twice a day for 2 weeks, and an appropriate analgesic such as ibuprofen 600 mg q4h is prescribed. In addition, the patient is referred to a physician to determine if a tetanus prophylaxis injection should be administered.
Since treatment of a patient with tooth avulsion is always an emergency, the following items should be available on notice (ie, on a prepared tray):
•Save-A-Tooth System (888-788-6684; Save-A-Tooth.com)
•Doxycycline capsules (100 mg)
•Orthodontic wire (0.06) and brackets
•Light-cured composite
•5-cc irrigating syringe
•Long-necked spoon excavator
•Emdogain (Biora.com). Periodontists and possibly oral surgeons are more likely to have this product in their office.
Post-reimplantation Stage
The patient should return 7 to 10 days after the reimplantation. At this appointment, the splint is checked for integrity and stability, and the tooth is anesthetized using local anesthesia. Endodontic therapy is performed in the usual fashion. A calcium hydroxide mixture such as Calasept (JS Dental) is placed in the cleansed canal, filling it completely.
CONCLUSION
Radiographic root resorption of avulsed teeth decreases dramatically when (1) the teeth are stored in an enclosed protective environment such as Save-A-Tooth and (2) the roots are conditioned prior to reimplantation. This improved success is due to a number of factors, including the recognition of different conditions of avulsed teeth and different approaches to treatment based on different clinical conditions. An appreciation of the importance of the residual PDL cells on the root surface has helped to reduce the incidence of root resorption following reimplantation of avulsed teeth.
Reference
1. Krasner P, Rankow H. New philosophy for the treatment of avulsed teeth. Oral Surg Oral Med Oral Pathol. 1995;79:616-623.
2. Matsson L, Andreasen JO, Cvek M, et al. Ankylosis of experimentally reimplanted teeth related to extra-alveolar period and storage environment. Pediatr Dent. 1982;4:327-9.
3. Cvek M, Granath LE, Hollender L. Treatment of non-vital permanent incisors with calcium hydroxide: III. Variation of occurrence of ankylosis of reimplanted teeth with duration of extra-alveolar period and storage environment. Odont Revy. 1974;25:43-6.
4. Cvek M, Cleaton-Jones P, Austin J, et al. Effect of topical application of doxycycline on pulp revascularization and periodontal healing in reimplanted monkey incisors. Endod Dent Traumatol. 1990;6:170-6.
5. Andreasen J.O. Relationship between cell damage in the periodontal ligament after replantation and subsequent development of root resorption. A time-related study in monkeys. Acta Odontol Scand. 1981;39:15-25.
6. Andreasen J.O, Reinholdt I, Dybdahl R, et al. Periodontal and pulpal healing of monkey incisors preserved in tissue culture before replantation. Int J Oral Surg. 1978;7:104-112.
7. Trope M, Friedman S. Periodontal healing of replanted dog teeth stored in ViaSpan, milk and Hank’s Balanced Salt Solution. Endod Dent Traumatol. 1992;8:183-8.
8. Lindskog S, Pierce AM, Blomlof L, et al. The role of the necrotic periodontal membrane in cementum resorption and ankylosis. Endod Dent Traumatol. 1985;1:96-101.
9. Lenstrup K, Skieller V. A follow-up study of teeth replanted after accidental loss. Acta Odontol Scand. 1959;17:503-509.
10. Kemp WB, Phillips J. Evaluation of 71 replanted teeth. J Endodon. 1977;3:30-35.
11. Andreasen JO, Hjorting-Hansen E. Replantation of teeth. I. Radiographic and clinical study of 110 human teeth replanted after accidental loss. Acta Odontol Scand. 1966;24:287-306.
12. Andreasen JO. Effect of extra-alveolar period and storage media upon periodontal and pulpal healing after replantation of mature permanent incisors in monkeys. Int J Oral Surg. 1981;10:43-53.
13. Blomlof L. Milk and saliva as possible storage media for traumatically exarticulated teeth prior to replantation. Swed Dent J. 1981;Suppl. 8.
14. Blomlof L, Otteskog P, Hammarstrom L. Effect of storage in media with different ion strengths and osmolalities on human periodontal ligament cells. Scand J Dent Res. 1981;89:180-187.
15. Lindskog S, Blomlof L. Influence of osmolality and composition of some storage media on human periodontal ligament cells. Acta Odontolog Scan. 1982;40:435-43.
16. Trope M. Clinical management of the avulsed tooth: present strategies and future directions. Dent Traumatol. 2002;18:1-11.
17. Andreasen JO. Exarticulations in “traumatic injuries of the teeth.” 2nd ed. Copenhagen, Denmark: Mungsgaard, 1981.
18. Pathways of the Pulp, Cohen S, Burns R. eds. Trope M. Traumatic Injuries Chapt 16, p 636-37 8th Ed. Mosby, 2002 St. Louis.
19. Andreasen JO, Reinholdt J, Riis I, et al. Periodontal and pulpal healing of monkey incisors preserved in tissue culture before replantations. Int J Oral Surg 1978;7:104-10.
20. Krasner P, Person P, Preserving avulsed teeth for replantation. J Am Dent Assoc 1992;123:80-88.
21. Haracz OM, Carnes DL, Walker WA. Determination of periodontal ligament cell viability in the oral rehydration fluid Gatorade and Milks of varying fat content. J Endodon 1997;23:687-90.
22. Andreasen JO, Andreasen FM. Essentials of traumatic injuries of the teeth, ed2, Copenhagen Denmark, 2000, Munksgaard International Publishers.
23. Courts M, Mueller WA, Tabeling JH. Milk as in interim storage medium for avulsed teeth. Pedatr Dent 1983;5:183-6.
24. Kling M, Cvek M, Mejare I. Rate and predictability of pulp revascularization in therapeutically reimplanted permanent incisors. Endo Dent Traumatol 1986;2:83-8.
25. Filippi, A, Pohl Y, von Arx T. Treatment of replacement resorption with Emdogain preliminary results after 10 months. Dent Traumatol 2001;17:134-138.
26. Iqbal MK. Effect of Emdogain on periodontal healing after replantation of permanent incisors in dogs. Dent Traumatol 2001;1736-45.
27. Andreasen JO. Luxation of permanent teeth due to trauma: a clinical and radiographic follow-up of 189 injured teeth. Scand J Dent Res 1970;78:273-8.
Dr. Krasner is in private practice of endodontics in Pottstown, Pa. He is a professor in the Department of Endodontology at Temple University, School of Dentistry. He is a co-author with Dr. Sam Seltzer of the second edition of the textbook “Endodontology: Biologic Aspects of Endodontics.” He can be received at endsurg@aol.com.