Since August 2001, when Dentistry Today presented a series of articles on emergency cardiovascular care and the use of automated external defibrillators (AEDs), AEDs have become widely available for emergency use in the public sector. This 2-part series provides the most current information on the rationale and use of AEDs. Part 1 discusses types of cardiac emergencies, factors necessary for successful resuscitation from cardiac arrest, and the rationale behind the concept of early defibrillation. Part 2 will discuss available AEDs, how they are used, and their potential importance in dental practice.
 |
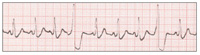 |
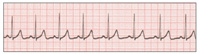
| Figure 1. Normal sinus rhythm (NSR). | Figure 2. Premature ventricular contractions (PVCs). |
 |
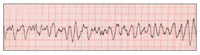 |
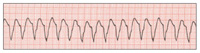
| Figure 3. Ventricular tachycardia (V-tach or VT). | Figure 4. Coarse ventricular fibrillation (VF). |
 |
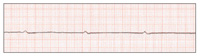 |
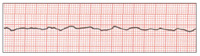
| Figure 5. Fine ventricular fibrillation. | Figure 6. Myocardial contraction ceases (asystole, “silent heart”). |
SUDDEN CARDIAC ARREST
Fifty percent of people in Western society with serious coronary artery disease (CAD) experience their first signs of the disease in a dramatic way—sudden cardiac arrest.7 The first sign of a progressive narrowing of the coronary arteries from a decades-long development of an atheroma (intra-arterial plaque) can be a rapid sequence of plaque rupture or erosion and formation of an occluding thrombus. This arterial obstruction leads to ischemia, an irritable myocardium, a sudden generation of ventricular fibrillation, collapse, and death. Whether the victim lives or dies at this point depends on whether the collapse has been witnessed; whether the people who respond are trained in basic life support, resuscitation, and defibrillation; and whether they access an emergency response system that can bring about early arrival of BLS (basic life support) and ACLS (advanced cardiovascular life support) resources.8
ACUTE CORONARY SYNDROMES (INCLUDING ACUTE MYOCARDIAL INFARCTION)
Though the mortality rate from coronary heart disease has steadily declined in the United States for the past 30 years, it remains the number one cause of death.7 Acute myocardial infarction (AMI, aka “heart attack”) occurs in approximately 1,100,000 Americans annually.1 As described above, an AMI usually develops when a plaque ruptures and a blood clot forms in a coronary artery, compromising blood flow to a portion of myocardium—most commonly located in the left ventricle. Deprived of blood, these now ischemic myocardial cells can no longer function normally (contracting synchronously with other myocardial cells) to pump blood out of the heart into the systemic circulation. The heart has been weakened. The victim of an AMI usually retains consciousness, but exhibits symptoms associated with decreased cardiac output as well as complaining of retrosternal pain, described variably as crushing, burning, and constricting “like there is a heavy weight on my chest” and exhibiting the classical radiation patterns associated with AMI (eg, upper epigastric region, left arm, left neck, left mandible).
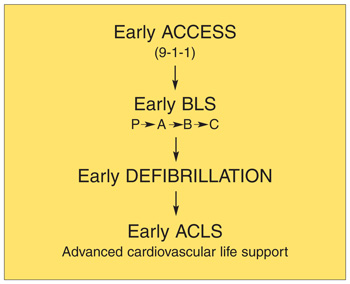 |
| Figure 7. The adult chain of survival. |
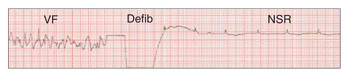 |
| Figure 8. Defibrilation converts VF to a NSR. |
 |
AMI MAY PROGRESS TO CARDIAC ARREST
From 4% to 18% of AMI’s progress to cardiac arrest.12 Ischemia makes the myocardium irritable and more likely to depolarize prematurely, provoking irregularities in the rhythm of the heart. Frequently noted at this time are premature ventricular contractions (PVCs) (Figure 2). In a PVC, the ischemic myocardium depolarizes prematurely, before the ventricles have refilled with blood following the previous contraction. No blood is ejected from the heart into the systemic circulation with a PVC, therefore no peripheral pulse is palpable. Cardiac output decreases and the symptoms described above increase in intensity. Since the majority of heartbeats are still normal, consciousness is retained, although at a diminished level (eg, “dizzy,” “lightheaded,” “faint”).
SUDDEN CARDIAC DEATH
Fifty-two percent of deaths associated with acute coronary syndromes, including AMI, occur within the first hour following the onset of symptoms, prior to the victim reaching the hospital.7 In 17% of patients, ischemic pain is the first, last, and only symptom.16 Seventy to 80% of cardiac arrests occur in the home.17
SURVIVAL FROM OUT-OF-HOSPITAL CARDIAC ARREST
In the United States, fewer than 5% of victims of out-of-hospital cardiac arrest are resuscitated and survive to be discharged from the hospital neurologically intact.15 Restoration of a perfusing cardiac rhythm requires prompt implementation of BLS followed by defibrillation within a few minutes of the initial arrest. As noted, from the time of collapse until defibrillation, survival rates decrease at about 7% to 10% per minute. When defibrillation is delayed, survival rates decrease to approximately 50% at 5 minutes, 30% at 7 minutes, approximately 10% at 9 to 11 minutes, and 2% to 5% beyond 12 minutes.23,24
References
- 2002 Heart and Stroke Statistical Update. Dallas, Tex: American Heart Association; 2002.
- Caffrey SL, Willoughby PJ, Pepe PE, et al. Public use of automated external defibrillators. New Engl J Med. 2002;347:1242-1247.
- State-specific mortality from sudden cardiac death–United States, 1999. MMWR Morb Mortal Wkly Rep. 2002;51:123-126.
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473-1482.
- White RD, Hankins DG, Bugliosi TF. Seven years’ experience with early defibrillation by police and paramedics in an emergency medical services system. Resuscitation. 1998;39:145-151.
- Becker LB, Pepe PE. Ensuring the effectiveness of community-wide emergency cardiac care. Ann Emerg Med. 1993;22:354-365.
- Gillum RF. Trends in acute myocardial infarction and coronary heart disease death in the United States. J Am Coll Cardiol. 1994;23:1273-1277.
- International Consensus on Science. Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2000;102(suppl):I-5-6.
- Calle PA, Verbeke A, Vanhaute O, et al. The effect of semi-automatic external defibrillation by emergency medical technicians on survival after out-of-hospital cardiac arrest: an observational study in urban and rural areas of Belgium. Acta Clin Belg. 1997;52:72-83.
- Malamed SF, Handbook of Medical Emergencies in the Dental Office. 5th ed. St. Louis, Mo: CV Mosby;2000: 000.
- Tan WA, Moliterno DJ. Aspirin, ticlopidine, and clopidogrel in acute coronary syndromes: underused treatments could save thousands of lives. Cleve Clin J Med. 1999;66:615-618,621-624,627-628.
- Antiplatelet Trialist’ Collaboration. Collaborative overview of randomized trials of antiplatelet therapy, part I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Brit Med J. 1994;308:81-106.
- Kern KB, Hilwig RW, Berg RA, et al. Efficacy of chest compression-only BLS CPR in the presence of an occluded airway. Resuscitation. 1998;39:179-188.
- Eisenberg MS, Hallstrom AP, Copass MK, et al. Treatment of ventricular fibrillation: emergency medical technician defibrillation and paramedic services. JAMA. 1984;251:1723-1726.
- Weaver WD, Copass MK, Bufi D, et al. Improved neurologic recovery and survival after early defibrillation. Circulation. 1984;69:943-948.
- Kannel WB, Schatzkin A. Sudden death: lessons from subsets in population studies. J Am Coll Cardiol. 1985;5(suppl):141B-149B.
- Litwin PE, Eisenberg MS, Hallstrom AP, et al. The location of collapse and its effect on survival from cardiac arrest. Ann Emerg Med. 1987;16:787-791.
- Rosomoff HL, Kochanek PM, Clark R, et al. Resuscitation from severe brain trauma. Crit Care Med. 1996;24:S48-S56.
- Cummins RO, Ornato JP, Thies WH, et al. Improving survival from sudden cardiac arrest: the “chain of survival” concept: a statement for health care professionals from the Advanced Cardiac Life Support Subcommittee and the Emergency Cardiac Care Committee, American Heart Association. Circulation. 1991;83:1832-1847.
- Stapczynski JS, Svenson JE, Stone CK. Population density, automated external defibrillator use, and survival in rural cardiac arrest. Acad Emerg Med. 1997;4:552-558.
- Cummins RO. EMT-defibrillation: national guidelines for implementation. Am J Emerg Med. 1987;5:254-257.
- Newman MM. The survival advantage: early defibrillation programs in the fire service. J Emerg Med Serv. 1987;12:40-46.
- Eisenberg MS, Horwood BT, Cummins RO, et al. Cardiac arrest and resuscitation: a tale of 29 cities. Ann Emerg Med. 1990;19:179-186.
- McIntyre KM. Cardiopulmonary resuscitation and the ultimate coronary care unit [editorial]. JAMA. 1980;244:510-511.
- Larson MP, Eisenberg MS, Cummins RO, et al. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Ann Emerg Med. 1993;
22:1652-1658. - Cobb LA, Fahrenbruch CE, Olsufka M, et al. Changing incidence of out-of-hospital ventricular fibrillation, 1980-2000. JAMA. 2002;288:3008-3013.
- International Consensus on Science. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. 2000:I-61.
- Lombardi G, Gallagher E, Gennis P. Outcome of out-of-hospital cardiac arrest in New York City: The pre-hospital arrest survival evaluation (PHASE) study. JAMA. 1994;271:678-683.
- Becker LB, Ostrander MP, Barrett J, Kondos GT. Outcome of CPR in a large metropolitan area—where are the survivors? Ann Emerg Med. 1991;20:355-361.
- Van Camp SP, Peterson RA. Cardiovascular complications of cardiac rehabilitation programs. JAMA. 1986;256:1160-1163.
- Nichol G, Hallstrom AP, Kerber R, et al. American Heart Association report on the Second Public Access Defibrillation Conference, April 17-18, 1997. Circulation. 1998;97:1309-1314
- Mosesso VN Jr, Davis EA, Auble TE, et al. Use of automated external defibrillators by police officers for treatment of out-of-hospital cardiac arrest. Ann Emerg Med. 1998;32:200-207.
- O’Rourke MF, Donaldson E, Geddes JS. An airline cardiac arrest program [see comments]. Circulation. 1997;96:2849-2853.
- Caffrey SL, Willoughby PJ, Pepe PE, et al. Public use of automated external defibrillators. N Engl J Med. 2002;347:1242-1247.
- BLS for Healthcare Providers. Dallas, Tex: American Heart Association; 2001:92.
Dr. Malamed is a professor of anesthesia and medicine at the School of Dentistry at the University of Southern California.