One of the major challenges of clinical dentistry is the management of pain and anxiety. The two are closely related. Fear of dental treatment is often the result of inadequate pain control. Pain control is most commonly achieved with local anesthesia. Nevertheless, Weinstein et al1 reported the incidence of failure of local anesthesia to be as high as 26.4%; 13% of patients reported the absence of anesthesia for tooth preparation; and 2.2% of patients reported they were unable to tolerate treatment. They suggested that fear was correlated with anesthetic failure.
About half of the US population does not receive yearly dental care. Between 6% and 14% of these individuals avoid treatment because of fear.2 This phenomenon is not unique to the United States. Similar statistics have been reported in Sweden and Japan.2,3,4 In an attempt to help patients manage fear and anxiety, many practitioners utilize nitrous oxide and oxygen analgesic. It is estimated that approximately 50% of dentists have the equipment needed to administer nitrous oxide, and more than 424,000 dental personnel are exposed to trace amounts of the gas as a result.5
Many dental schools and dental hygiene schools urge students to have a very cautious attitude toward the use of nitrous oxide. This is a result of reports concerning the effect of trace amounts of gas on office personnel, particularly on pregnant women.6-8 This paper discusses the issue of safety of nitrous oxide for dental personnel.
HISTORY OF THE USE OF NITROUS OXIDE
Nitrous oxide was discovered by Joseph Priestley in 1776. Humphry Davey wrote about the effect of nitrous oxide on diminution of pain associated with extraction of his third molar. In the 1840s Gardner Quincy Colton, a chemist and intinerant lecturer, introduced nitrous oxide to the public after reading Davey’s account. Colton introduced nitrous oxide to Horace Wells, a dentist who was the first to use gas inhalation for relief of pain. On December 11, 1844 Wells had a local dentist, Dr. John Riggs, extract one of Wells’ teeth while under the effects of nitrous oxide.9
Following this, nitrous oxide was used in dentistry as a general anesthetic until the advent of local anesthesia. With local anesthesia, dentistry had another option for controlling pain. In the early part of the twentieth century, nitrous oxide was reported as a sedative/analgesic. In the 1950s Langa and others popularized its use as an antianxiety agent by demonstrating that it could be used as a sedative in conjunction with local anesthesia. If the percentage of gas delivered was limited to 25% to 40%, few side effects were seen.10
OCCUPATIONAL SAFETY
Occupational exposure to certain chemicals, materials, and ionizing radiation has been shown to cause serious health effects. As examples, liver disease is a toxic effect of exposure to carbon tetrachloride in the dry-cleaning industry; benzedrine has been associated with bladder cancer in the chemical industry; and occupational exposure to x-rays has been associated with a variety of problems including cancer and spontaneous abortions.11
The first indication that trace amounts of anesthetic gas might be associated with side effects was a report in 1968 by Vaisman12 who reported problems in Russian female anesthesiologists, including irritability, headache, fatigue, nausea, pruritis, and spontaneous abortion. Studies by Askrog and co-workers13 indicated that women who worked in operating rooms faced similar problems. In 1956 Lassen and colleagues14 observed that nitrous oxide was associated with pronounced bone marrow depression when patients were exposed to 50% nitrous oxide for 14 days. The marrow returned to normal within a few days after treatment was stopped.
ANIMAL STUDIES
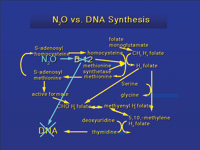 |
| Figure 1. Nitrous oxide deactivates vitamin B12. Two tests can be performed to determine the level of vitamin B12 suppression. Methionine synthetase and deoxyuridine levels can be measured and are an indication of the effect of nitrous oxide exposure. |
Anesthetic agents have been shown to be associated with teratogenic effects in animal studies.15 These effects were observed at low concentrations. Eger7 demonstrated teratogenic changes, fetal skeletal changes, reduced fetal weight, and miscarriage when rats were exposed to nitrous oxide during pregnancy. Abdul-Kareem and co-workers16 exposed rats to nitrous oxide at 50, 500, or 5,000 parts per million (ppm) for 6 hours a day, 5 days a week, for 2 and 13 weeks, and showed changes in neurotransmitters. In a similar study, Healy and colleagues17 exposed mice to 50, 500, or 5,000 ppm for 5 days a week for 2 or 13 weeks. The weight of the liver was lower for all three concentrations at 13 weeks, but not at 2 weeks. The number of circulating leukocytes was lower at the highest concentration at 2 weeks, and at all concentrations at 13 weeks. The bone marrow also demonstrated a dose-related reduction in deoxyuridine uptake. Fujinaga et al18 demonstrated rat fetal mortality when pregnant rats were exposed to 75% nitrous oxide. Viera and co-workers19 demonstrated spontaneous abortions in rats with exposure to nitrous oxide at 1,000 ppm. Similarly, testicular damage was observed after a minimum of 2 days exposure to 20% nitrous oxide,20 and rat bone marrow was depressed with exposure to 80% nitrous oxide,21 but not at 1% exposure even after 6 months.22 Furthermore, nitrous oxide at 1,000 ppm decreases the vitamin B12 component of the enzyme methionine synthetase by interfering with folate metabolism, and this can impair DNA synthesis23 (Figure 1).
In a study intended to simulate operating room conditions, rats were exposed to 1 ppm halothane and 50 ppm nitrous oxide, or 10 ppm halothane and 500 ppm nitrous oxide for 7 hours a day, 5 days a week, for 104 weeks. No increase in tumor incidences was found.24,25 Several studies showed increased fetal mortality or small litter size when rats were exposed to 1,000 ppm nitrous oxide if the rats were exposed to this concentration for a number of days. No effect was observed at lower concentrations.19 In addition, studies have failed to demonstrate teratogenic effects for rats or mice exposed to 0.5%, 5%, or 50% nitrous oxide for 8 hours a day throughout gestation.26 In summary, the animal studies suggest potential problems associated with exposure to nitrous oxide. They also indicate that if the exposure levels are low, problems can be lessened or not observed.
RETROSPECTIVE HUMAN STUDIES
The dental office is an ideal location to study the effects of occupational exposure to nitrous oxide as some offices use nitrous oxide, and others do not. Cohen and colleagues,27,28 conducted two important studies demonstrating changes associated with higher levels of exposure to nitrous oxide. These studies demonstrated a higher incidence of hepatic, renal, and neuralgic disorders among exposed personnel. In addition, there was an increase in spontaneous abortions seen among chairside assistants and the dentists’ wives. There was also an increase in congenital abnormalities in children of assistants. There are, however, several problems associated with these studies. There is concern that respondents might not have accurately recalled events in the past. Furthermore, 18.7% of the respondents were also exposed to other anesthetic gases, and no attempt was made to identify recreational users of nitrous oxide.29 These studies did identify a problem for pregnant females who worked in dental offices where nitrous oxide was used. The studies did not suggest at which level exposure became a problem.
OCCUPATIONAL SAFETY AGENCIES
There are three governmental agencies responsible for establishing appropriate levels of exposure to chemicals: the American Conference of Governmental Industrial Hygienists (ACGIH), Occupational Safety and Health Administration (OSHA), and the National Institute for Occupational Safety and Health (NIOSH). The ACGIH publishes a set of suggested maximum exposure levels for many chemicals. It is clear in their literature that these levels are only suggestions and guidelines to be used as a guide by industrial hygienists, and they should not be used to set regulations. OSHA sets minimum guidelines for employers to assure a safe workplace in the United States. NIOSH is the occupational safety and health research arm of the federal CDC that makes recommendations to OSHA.30 The NIOSH publication, Alert, suggests that the recommended exposure limit is 25 ppm on a time-weighted average (usually an average exposure over an 8-hour day of 25 ppm). These levels were recommended based on two studies by Bruce.31,32
THE BRUCE STUDIES
In the first study, effects were seen when subjects were exposed to 50 ppm nitrous oxide with 1 ppm halothane, but not to 500 ppm nitrous oxide. Changes were seen in audiovisual tasks, tachistoscopic tasks, memory passages, and digit span tests.31 The second study showed a slight effect when subjects had been exposed to 500 ppm nitrous oxide for 4 hours, and were asked to recall a series of numbers and were given a digit span test.32 It is important to note that in two letters Bruce later claimed this study was flawed.33,34 He stated that the conclusions of the study were wrong, derived from data subject to sampling bias and not applicable to the general population. He called for the NIOSH standards to be revised.
Many papers have been published in the dental literature which mention the effect of nitrous oxide on motor skills, but this effect has not been demonstrated for nitrous oxide acting alone. Furthermore, no study has been published that demonstrates a detrimental effect of 50 ppm nitrous oxide. One study demonstrated an effect at 500 ppm, but it has been retracted by the author because neither he nor others have been able to duplicate the findings.31,32 In spite of these problems with the data, the NIOSH standards have not been revised.
WHAT IS A SAFE LEVEL OF EXPOSURE?
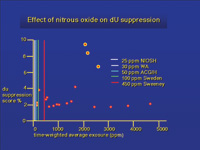 |
| Figure 2. The Sweeney et al study measured deoxyuridine suppression. They suggested that 450 ppm of nitrous oxide was a safe occupational level. This is much higher than what has been adopted. |
Ahlborg35 reported that Swedish midwives exposed to nitrous oxide did not have difficulty becoming pregnant unless they were exposed to nitrous oxide 30 or more times a month. In another study of midwives, he did not observe an increase in spontaneous abortions with exposure to nitrous oxide, but this was seen with night shifts and increased work load and no nitrous oxide exposure.36 Sweeney37 studied 20 practicing dentists by having them wear a nitrous oxide monitor. At the conclusion of the study, bone marrow samples were collected. The exposures ranged from 50 ppm to more than 5,000 ppm on a time-weighted average. All but three participants were above 400 ppm. Bone marrow depression was seen in three dentists with exposures over 1,800 ppm. Some participants showed no change even with an exposure of over 5,000 ppm. To set levels that would assure safety, Sweeney suggested exposure should not exceed 450 ppm on an 8-hour time-weighted average (Figure 2). Nevertheless, Nunn38 showed that 450 ppm of nitrous oxide exposure had no effect on operating room personnel or experimental animals. In addition, Karakaya and colleagues39 concluded that chronic exposure to halothane and nitrous oxide does not affect serum immunoglobulin levels, white blood cell counts, or lymphocyte subpopulations of anesthetists, anesthetic assistants, or anesthetic nurses.
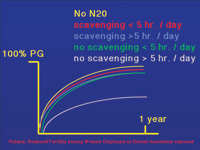 |
| Figure 3. The Roland et al study showed no change in ability of dental assistants to become pregnant provided scavenging is used or the office uses nitrous oxide on a limited basis. |
Rowland et al8 examined pregnancy in female dental assistants (facundability). They first looked at exposure to mercury vapor and found no effect. They reevaluated the data, taking into consideration whether the assistants had been exposed to nitrous oxide. The assistants were divided into five groups: a control group that was not exposed to nitrous oxide; two groups in offices that used scavenging devices to remove traces of nitrous oxide and had either an exposure of less than 5 hours per week or greater than 5 hours per week; and two groups with no scavenging devices and again grouped by high or low usage. Only the group with no scavenging devices and high utilization had a statistically significant decrease in facundability (Figure 3). These studies strongly suggest that if scavenging is used and nitrous oxide levels are kept below 450 ppm, the dental office staff is not at risk.
HOW CAN ENVIRONMENTAL EXPOSURE BE REDUCED?
 |
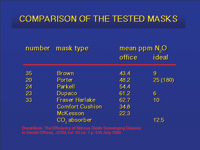 |
| Figure 4. The Brown Mask results in levels of exposure in the 50 ppm range. This led to the suggestion by OSHA that 50 ppm should be the goal. Suction is applied to the space between masks to scavenge any gas that may escape around the seal. | Figure 5. Donaldson and Orr showed that scavenging devices were effective in reducing the trace levels of nitrous oxide. |
Donaldson and Orr have shown that many scavenging devices being used in dental offices can result in environmental levels in the 40 to 60 ppm range.40 (Figures 4, 5) Nevertheless, NIOSH has stated that exposure should be limited to 25 ppm, and if this level of exposure could not be achieved then self-contained respirators were required.6
It is clear that dental offices using nitrous oxide analgesia should have one of the available scavenging systems on each machine. The systems that deliver the gases come in three basic forms: semi-open, open, and semi-closed systems.
Semi-open Systems
 |
 |
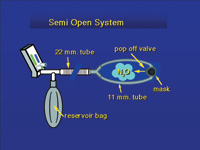
| Figure 6. The semi-open system allowed gas to escape from a pop-off valve on the mask and into the breathing space of the dentist and dental assistant. This led to nitrous oxide levels as high as 5,000 ppm. | Figure 7. Some older masks had pop-off valves that directed exhaust gases into the breathing area of the dentist. |
Prior to scavenging, all systems could best be described as semi-open systems (Figure 6). The gas left the machine and went to a connector attached to a hose and a reservoir bag. The hose carried gas to a Y-shaped connector and two smaller hoses that went around the patient’s head and attached to either side of the nose mask. The nose mask had a pop-off valve that vented exhaust gas into the dental operatory (Figure 7).
Open Systems
Some machine manufacturers placed a one-way valve downstream from the reservoir bag. With this valve, the system was now fully open and permitted no rebreathing. All exhaled gas was blown out through the valve of the mask into the breathing zone of the dentist and assistant who were treating the patient. With open and semi-open systems, trace gas levels have been reported as high as 5,000 ppm of nitrous oxide in the dentist’s and assistant’s breathing zone.41
Semi-closed Systems
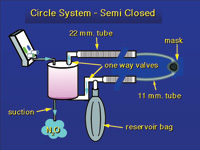 |
| Figure 8. Semi-closed or circle system with a CO2 absorbing canister. The gas is reused, removing CO2 each time the gas passes through the soda lime granules in the absorber canister. This system requires a nitrous oxide machine that can deliver low flow volumes. |
A third system that is common in hospital operating rooms is the semi-closed, circle system. Here a CO2 absorbing canister is placed in the system. The patient inhales, pulling gas from the reservoir bag through the CO2 absorber, thus removing CO2. The gas goes through a hose to one side of the mask. There is a one-way valve that prevents the exhaled gas from returning back down this tube. The tube on the other side of the mask carries the exhaled gas back to the reservoir bag where the cycle starts again. Each pass through the CO2 absorber removes the CO2 produced by the patient and exhaled (Figure 8). These systems have a pop-off valve to allow excess gas to escape. Suction is applied to this valve to remove the exhaust gases from the room.
CURRENT SCAVENGING SYSTEMS
 |
 |
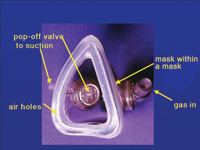
| Figure 9. This mask has a vacuum applied between the two shells. Exhaled gas enters the space between the two shells and is removed from the space by suction. | Figure 10. Mask insert for a Brown mask system. Suction is applied to the space between the masks. |
Today’s scavenging systems are predominantly open systems with suction placed at the pop-off valve on the mask. Some systems also attempt to remove the air that is around the mask, which may contain traces of nitrous oxide that the patient has exhaled through their mouth or that has leaked from around the mask (Figure 9). One of the earliest (and still excellent) systems is a mask within a mask, or the Brown Mask. The space between the inner and outer masks is under vacuum (Figure 10). Another current system has a mask with a disk that sits over the pop-off valve. The plastic disk (about 3 inches in diameter) removes the exhaust gas from the pop-off valve and brings in surrounding air via a slit in the disk. Unfortunately, this mask is rather noisy.
 |
 |
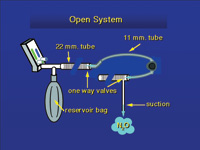
| Figure 11. In the open system, gas is drawn from the bag and exhaled through a second hose, or into a space around the mask. Suction then removes the waste gas. | Figure 12. By adding a hose, two one-way valves, and suction, a semi open system can be converted to a scavenging system. |
In one current and inexpensive system (Figures 11 and 12), the hose from the bag goes to one side of the mask. The mask has no pop-off valve. Exhaled gas leaves the mask from the opposite side and goes into a second hose that is approximately 1 m long. There is a one-way valve that only allows gas to flow away from the patient. The end of this hose is open to the air, but suction is applied to the hose just downstream from the one-way valve, and the exhaled gas is removed. The suction from a typical saliva ejector that is part of a high-volume suction system is adequate. In practice, there is fresh air flowing into the open end of the exhaust hose, and this is removed with the exhaled gas. All the systems now on the market can be expected to keep levels of nitrous oxide in the 50 ppm range.40
Conclusion
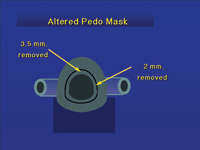 |
| Figure 13. By altering the shape and size of the nose hole, a pediatric mask can be used by adults. |
Every nitrous oxide/oxygen machine must have a scavenging system with adequate suction. Furthermore, there must be a reasonable exchange of air in the operatory. Outside air should be brought into the office by heating and cooling systems. This will help clear trace amounts of gas, and it is suggested that the minimum air exchange is 5 per hour, with 15 to 20 changes being better. Hoses and connectors should be routinely checked for leaks, and masks should be selected that fit the patient (Figure 13). The patient should also be discouraged from speaking while using nitrous oxide sedation. If all these suggestions are followed, trace amounts of nitrous oxide will be in the 50 ppm range.
References
1. Weinstein P, Milgrom P, Kaufman E, et al. Patient perceptions of failure to achieve optimal local anesthesia. Gen Dent. 1985;5/6:218-220.
2. Milgrom P, Weinstein P, Kleinknecht, et al. Treating Fearful Dental Patients. Reston, Virginia: Reston Publishing Co Inc; 1985.
3. Friedaon E, Feldman J. The public looks a dental care. J Am Dent Assoc. 1953;57:325-335.
4. Kleinknecht R, McGlynn F, Thorndike R, et al. Factor analysis of the Dental Fear Survey with cross validation. J Am Dent Assoc. 1984;108:59-61.
5. Domoto P, Weinstein P, Melnick Sl, et al. Results of a dental fear survey in Japan: implications for dental public health in Asia. Comm Dent Oral Epidemiol. 1988;16:199-201.
6. National Institute of Occupational Safety and Health. NIOSH Alert: Request for Assistance in Controlling Exposures to Nitrous Oxide During Anesthetic Administration. Washington, DC: United States Department of Health and Human Service; 1994.
7. Eger EL. Fetal injury and abortion associated with occupational exposure to inhaled anesthetics. AANA J. 1991;59:309-312.
8. Rowland AS, Baird DD, Wienberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327:993-997.
9. Langa H. Relative Analgesia in Dental Practice. Philadelphia, Pa: WB Saunders Co; 1968.
10. Jastak JT. Pharmacosedation. CDA J. 1993;2:27-32.
11. Jastak JT, Greenfield W. Trace contamination of anesthetic gases: a brief review. J Am Dent Assoc. 1997;96:758-762
12. Vaisman AI. Working conditions in surgery and their effect onthe health of anesthesiologists. Anesthesiol. 1968;29:565.
13. Askrog V, Petersen R. Contamination of operating theatres by inhalation anesthetics and ionizing radiation. Nord Med. 1970;83:501-504.
14. Lassen HAC, et al. Treatment of tetanus: Severe bone marrow depression after prolonged nitrous oxide anesthesia. Lancet. 1:527-530.
15. Askrog V, Harvald B. Teratogenic effects of inhalation anesthetics. Nord Med. 1970;86:498-500.
16. Abdul-Kareem HS, Sharma RP, Drown DB. Effects of repeated intermittent exposures to nitrous oxide on central neurotransmitters and hepatic methionine synthetase activity in CD-1 mice. Toxicology Industrial Health. 1991;7:97-108.
17. Healy CE, Drown DB, Sharma RP. Short-term toxicity of nitrous oxide an the immune, hemopoetic, and endocrine system in CD-1 mice. Toxicology Industrial Health. 1990;6:57-70.
18. Fujinaga M, Baden JM, Shepard TH, et al. Nitrous oxide alters body laterality in rats. Teratology. 1990;41:131-135.
19. Viera E, Kleaton-Jones P, Austin JC, et al. Effects of low concentrations of nitrous oxide on rat fetuses. Anesth Analgesia. 1980;59:175-177.
20. Kripke, BJ, Kelman AD, Shah NK, et al. Testicular Reaction to Prolonged Exposure to Nitrous Oxide. Anesthesiol. 1976;44:104-113.
21. Green CD, Eastwood DW. Effects of nitrous oxide inhalation on hematopoesis in rats. Anesthesiol. 1963;24:341.
22. Cleaton-Jones P, et al. Effect of intermittent exposure to a low concentration of nitrous oxide on hematopoesis in rats. Anesthesiol. 1977;49:233.
23. Sharer NM, Nunn JF, Royston JP, et al. Effects of chronic exposure to nitrous oxide on methionine synthetase activity. Br J Anesth. 1983;55:693-701.
24. Coate WB, Ulland BM, Lewis TR. Chronic exposure to low concentrations of halothane-nitrous oxide: lack of carcinogenic effect in the rat. Anesthesiol. 1979;50:306-309.
25. Baden JM, et al. Carcinogen bioassay of nitrous oxide in mice. Anesthesiol. 1979;50:306.
26. Hardin RD, et al. Testing of selected workplace chemical for teratogenic potential. Scand J Work Environ Health. 1981;7:66.
27. Cohen EN, Brown BW, Wu ML, et al. Occupational disease in dentistry and chronic exposure to trace anesthetic gases. J Am Dent Assoc. 1980;101:21-31
28. Brodsky JB, Cohen EN, Brown BW Jr, et al. Exposure to nitrous oxide and neurologic disease among dental professionals.Anesth Analgesia. 1981:60:297-301.
29. Yagiela JA. Health hazards and nitrous oxide: a time for reappraisal. Anesth Prog. 1991;38:1-11.
30. American Dental Association. ADA seeks data on nitrous exposure. ADA News. November 23, 1992;23.
31. Bruce DL, Bach MJ. Effects of trace anaesthetic gases on behavioral performance of volunteers. Br I Anesth. 1976;48:871-876.
32. Bruce DL, Bach MJ, Arbit J. Trace anesthetic effects on perceptual, cognitive and motor skills. Anesthesiol. 1974;40:452-458.
33. Bruce DL. Recantation revisited. Anesthesiol. 1991;74:1160-1161.
34. Weinger MB, Englund CE. Ergonomic and human factors affecting anesthetic vigilance and monitoring performance in the operating room environment. Anesthesiol. 1990;73:995-1021
35. Ahlborg G, Axelsson G, Bodin L. Shift work, nitrous oxide exposure and subfertility among Swedish midwives. Int J Epidemiol. 1996;25:783-790.
36. Ahlborg G, Axelsson G, Bodin L, Shift work, nitrous oxide exposure, and spontaneous abortion among Swedish midwives. Occupational Environmental Med. 1996;53:374-378.
37. Sweeney B, Bingham RM, Amos RJ, et al. Toxicity of bone marrow in dentists exposed to nitrous oxide. Br Med J (Clin Res Ed). 1985;291:567-569.
38. Nunn JF, Sharer N, Royston D, et al. Serum methionine and hepatic enzyme activity in anaesthetists exposed to nitrous oxide. exposed to nitrous oxide. Br J Anesth. 1982;55:593-597.
39. Karakaya A, Tuncel N, Yucesoy B, et al. The effects of volatile anesthetic agents on human immune system function via occupational exposures. Immutiopharmacol Immuriotoxicol. 1992;14:251-259.
40. Donaldson D, Orr J. A comparison of the effectiveness of nitrous oxide scavenging devices. J Canadian Dent Assoc. 1989; 55:535-537.
41. Greenfield W. Potential hazards of chronic exposure to trace anesthetic gases: implications for dentistry. J Am Dent Assoc. 1980;101:158-159.
Dr. Quarnstrom has received fellowships in the Academy of General Dentistry, American Dental Society of Anesthesiology, and the International College of Dentists, and is a diplomate of the American Board of Dental Anesthesiology. He is a clinical assistant professor in the Department of Dental Public Health Sciences at the Univers










