More than a million new cases of cancer are diagnosed in the United States each year.1 In light of oral complications of cancer therapies, it is vital for the dental team to assume a more comprehensive role in the management of these patients. Optimal management of the cancer patient involves an interdisciplinary approach that requires assessment, communication, and interaction among the healthcare team during all stages of cancer therapy.
Timely and effective dental management of cancer patients is an area that both the medical and dental profession may overlook. When an individual is diagnosed with a life-threatening cancer that requires extensive therapy, the condition of the oral cavity may not be a priority. The patient is more concerned about survival and the implications of the prospective treatments. Nevertheless, oral complications from cancer therapies can seriously compromise patients’ health and quality of life, as well as affect their ability to complete the planned cancer treatment. Oral complications can be so severe that patients may not be able to tolerate the prescribed therapy and may either discontinue or postpone scheduled treatments.1 Therefore, it is paramount that the dentist is educated and prepared to treat these patients when they are seen in the office.
The 4 major types of cancer therapy that affect the oral cavity are head and neck irradiation, chemotherapy, blood and bone marrow transplant, and head and neck surgery.2 Oral complications occur in almost 100% of head and neck radiation patients, up to 75% of blood and bone marrow transplant recipients, and almost 40% of patients receiving chemotherapy.1
Common oral complications of cancer treatments include the following:
(1) Mucositis: this is marked by inflammation and ulceration of the mucosal lining of the oral cavity.3
(2) Infection: mylosuppression results in neutropenia, which makes the patient susceptible to bacterial, viral, and fungal infections.4
(3) Xerostomia: the subjective complaint of “dry mouth” results from a decrease in the production of saliva.5
Prolonged oral complications of radiation therapy include the following:
(1) Rampant caries: postradiation damage to the salivary glands creates an environment conducive to significant dental decay.6
(2) Trismus: this results from a loss of elasticity of the masticatory muscles, restricting normal opening of the mouth.1
(3) Osteoradionecrosis: these complications of radiation therapy are associated with hypovascularity and necrosis of bone followed by trauma-induced or spontaneous mucosal breakdown, leading to a nonhealing wound.7
The 3 stages of managing the cancer patient include diagnosis and pretherapy assessment, oral care during therapy, and oral care after completion of cancer therapy.
DIAGNOSIS AND PRETHERAPY ASSESSMENT
The pretherapy stage is that period of time from diagnosis of the cancer to the initiation of treatment. Patient education, optimal oral hygiene, adequate nutrition, and avoiding tobacco and alcohol are critical components in helping to prevent or minimize oral complications.1 A thorough dental examination should be conducted to include the following:
(1) Hard and soft tissues, including periodontal status and oral hygiene. The condition of existing restorations, tooth mobility, and tooth vitality are also evaluated.
(2) Previous oral trauma and history of mucocutaneous disorders such as herpes simplex infection, aphthous ulcers, and candidiasis.
(3) Observation of salivary function/flow by expressing saliva from the parotid duct.
(4) Obtaining appropriate radiographs (panoramic and intraoral bite-wing radiographs at a minimum).
(5) Coordinating a plan with the patient, family, and oncologist for treatment of emergent care as well as long-term maintenance.
The dentist should obtain the following information from the oncologist:
(1) The patient’s current health history, cancer diagnosis, and prognosis.
(2) If chemotherapy is planned, the anticipated number of treatment cycles and route of administration of the chemotherapeutic agents.
(3) A baseline complete blood count.
(4) If radiation is planned, the cumulative radiation dose, schedule, and site to be irradiated. The patient who receives a cumulative dose of more than 7,000 rad (70 Gy) has a signifi-cantly greater chance of developing osteoradionecrosis than patients receiving less than 5,000 rad (50 Gy).2
Patients should be counseled regarding expected oral complications from cancer therapy. A thorough dental prophylaxis and oral hygiene instruction to include the use of an extrasoft toothbrush with daily flossing is needed to minimize future oral problems. Denture patients should remove their dentures at night and soak them in a cleanser. Patients with re-movable appliances should be told to scrub the prosthesis daily with a denture brush. Sources of odontogenic infection should be eliminated. Nonrestorable fractured teeth, advanced caries, teeth with a poor periodontal prognosis, partially erupted third molars with the potential for pericoronitis, and teeth within the radiation field that have unresolved apical lesions should be considered for extraction. Ideally, extractions for patients undergoing radiation therapy should be performed about 3 weeks before the initiation of therapy, with the minimum being 14 days. For chemotherapy, it is recommended to allow 3 to 5 days in the maxilla, 5 to 7 days in the mandible, and 7 to 10 days for third molars.2
Conventional endodontic therapy should be accomplished on teeth outside the radiation field that present with necrotic or infected periapical lesions. It is important to take impressions during the pretherapy phase so that custom fluoride trays and/or occlusal splints to protect against a bruxism habit can be fabricated.2
DURING CANCER THERAPY
Cancer treatment usually lasts approximately 30 to 45 days, however, some individuals may receive chemotherapy for several years depending on the type of cancer and protocol used.2 A cycle of chemotherapy typically consists of administration over a 3-day to 5-day period, followed by immunosuppression marked by a decreased neutrophil count, and then recovery sufficient to proceed with the next course of chemotherapy. While elective dental treatment should be deferred during cancer therapy, there may be times when emergency treatment must be performed. If dental treatment is necessary during chemotherapy, the oncologist should be consulted and a complete blood count requested. The risk of infection and septicemia is greatest when the patient’s neutrophil count is below 1,000/mm3.8 A decreased platelet count can also occur as a result of chemotherapy, placing the patient at risk for hemorrhage. Emergency treatment should only be performed if the absolute neutrophil count is greater than 1,000/mm3 and the platelet count is greater than 50,000/mm3. Antibiotic prophylaxis and/or aggressive use of antibiotics should be considered for the neutropenic patient.8 If the patient has a central venous catheter for the delivery of chemotherapy, then the American Heart Association regimen for antibiotic prophylaxis should be implemented.1
The importance of good oral hygiene before, during, and after cancer therapy cannot be over-emphasized. In conjunction with a regimented oral hygiene program, the use of oral rinses has been proven to be quite effective. Alkaline saline (salt/bicarbonate) mouthrinses soothe and hydrate gingival and mucosal tissues, clean, lubricate tissues, remove debris, dissolve and dilute thick saliva, and neutralize acids.3 The use of chlorhexidine gluconate 0.12% has been recommended for many years to control plaque accumulation in patients undergoing cancer therapy. However, routine use is not advisable because of the possible emergence of Gram-negative bacilli infections in the oropharynx, the alcohol content may be irritating to patients with mucositis, and the potential for chlorhexidine to interfere with nystatin preparations during candidiasis treatment.9 Chemotherapeutic agents predominantly affect the rapidly dividing cells of the target tumor, resulting in mucositis and ulceration of the mucosal lining of the mouth, pharynx, and esophagus. The eroded or denuded surfaces may provide a port of entry for infectious organisms to the systemic circulation.2 Mucositis is usually associated with ulceration, pain, and bleeding. The patient may describe a burning sensation of the mucosa within 7 to 10 days of beginning chemotherapy. The initial clinical manifestation is erythema and inflammation. Over the next 3 to 5 days erosion and ulceration develop and persist for the next 2 weeks until the termination of chemotherapy.10
During radiation therapy, patients will typically develop mucositis during the second week of radiation therapy. The condition slowly subsides a few weeks after completion of radiation treatment.8 The inflammatory response of the radiation-induced mucositis varies in degree depending on the dosage, target area, and duration of treatment.4
Topical anesthetics and coating agents can be effective in providing symptomatic relief for oral mucositis. Diphenhydramine or Benadryl elixir (Parke-Davis) mixed 1:1 with Kaopectate (UpJohn) coats the mucosa and soothes the pain associated with mucositis. The patient should rinse and expectorate with this mixture every 2 hours.3 Viscous lidocaine can also be used as a coating agent. The patient will rinse for 30 seconds and expectorate.8 When these topical anesthetics are used, the patient should be warned not to swallow and should be aware of the possibility of an increased gag reflex.
The patient with chemotherapy-induced mucositis is at high risk for developing a secondary herpes simplex virus infection. Mucositis complicated by herpes infection tends to be more severe and has a longer duration. For leukemia patients as well as those re-ceiving a bone marrow transplant, reactivation of the herpes virus occurs in 50% to 80% of patients who are seropositive for herpes.2 Prophylactic acyclovir (Glaxo Wellcome) is often used in bone marrow transplant patients. However, it is not routinely used with patients who have chemotherapy-induced mucositis.2 The prophylactic regimen for acyclovir is 400 mg 2 times a day for up to a year and then re-evaluation. Patients with a prior history of herpes infection or a positive culture for herpes should be given an acute regimen as follows: acyclovir (200 mg) 5 times a day for 7 days.11
| Table. Preventive/Therapeutic Guide for the Cancer Patient. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Fungal or candidial infections of the oral mucosa are common in patients undergoing chemotherapy. Once established in the oral cavity, candidiasis has the potential for infecting the esophagus and becoming a systemic infection. Mild cases should respond to nystatin ointment or clotrimazole troches (Mycelex, Bayer). (See Table for regimen.) More severe infections or nystatin-resistant patients should be treated with amphotericin B, administered intravenously, to prevent systemic dissemination.8
POST-CANCER THERAPY
After chemotherapy, it is important to establish a regular recall schedule with a dental prophylaxis provided every 3 to 4 months. Before the commencement of routine dental treatment, it is necessary to confirm a normal hematological status. Due to immunosuppression, leukemia patients or bone marrow recipients are at continued risk of herpes infection. The use of prophylactic acyclovir may be indicated for these patients during the post-cancer therapy stage.2
 |
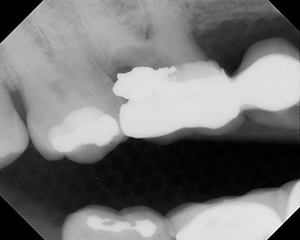
| Figure 1. Devastating effect of radiation-induced caries. |
Several long-term oral complications from radiation therapy may adversely affect the patient’s quality of life. Xerostomia is the most common and significant dental problem in irradiated patients. The severity depends on the radiation dosage, location, and the salivary glands that are exposed. Xerostomia can cause discomfort, affect the fit of removable prostheses, and make it difficult to chew and swallow. Radiation-induced xerostomia is rapid in onset, pronounced, and irreversible. Because xerostomia is almost certain to persist after therapy, appropriate management is paramount to prevent the progression of extensive dental caries.6 Figure 1 shows a 70-year-old African American male with a history of head and neck radiation status post 10 years. Only 4 visible teeth remain, and several other teeth are severely broken down with remaining tooth structure at or below the gingiva.
Treatment of radiation xero-stomia and accompanying discomfort includes replacement therapy and/or stimulation of salivary flow, caries prevention with antimicrobials and fluoride treatments, and the prevention of secondary fungal infections. Topical salivary substitutes moisturize the oral cavity. Oral Balance (Laclede) is a long-lasting lubricant gel that adheres to the mucosa and provides symptomatic relief from oral dryness for several hours. It contains the antimicrobial factors lactoferrin, lactoperoxidase, and lysozyme. Omni Plaque Fighter Spray (Omni Oral Pharmaceuticals) contains 1.2% poloxamer 407 dimeticone, an agent that coats and protects the mucosa for several hours.
Chewing gums with xylitol as a nonfermentable sweetener have shown encouraging results in reducing caries.12 Xylitol is a 5-carbon sugar alcohol that has demonstrated anticariogenic properties. Due to it’s nonfermentable property, xylitol-containing chewing gums such as Biotene (Laclede) can contribute to stabilizing the carious process.13
If functioning salivary gland tissue remains after radiation therapy, then prescribing salivary stimulants such as Pilo-carpine HCl (Ciba Vision) may be beneficial. Stimulated saliva contains antibacterial enzymes and buffering components that neutralize acids. The use of Pilocarpine HCl may eliminate or reduce the need for the patient to sip water frequently or use salivary substitutes. Patients may find it helpful to use Pilocarpine HCl 30 to 60 minutes before mealtime to assist in mastication.2 Pilocarpine HCl is available in solution 1 mg/cc, 3 tsp daily, or 5-mg tablets (Salagen), 3 to 4 tabs daily. Contraindications to Pilo-carpine HCl include uncontrolled asthma, narrow angle glaucoma, and allergy to the drug.10
Chlorhexidine gluconate 0.12% can be used to help reduce the level of Streptococcus mutans in patients receiving head and neck radiation.14 Treatment with chlorhexidine gluconate 0.12% consists of a 14-day regimen (bid), which is adequate to achieve a therapeutic endpoint. Any more than 2 weeks of treatment is not effective and therefore should be discouraged. Chlorhexidine gluconate is a highly effective antimicrobial rinse and will suppress S. mutans from 12 to 26 months.12
While chlorhexidine acts to decrease the microbial load initially, the daily and long-term use of fluoride is critical in preventing caries in patients receiving radiation therapy. Neutral sodium fluoride and stannous fluoride products are commonly used for home care. Most 0.4% SnF2 gels have a pH of about 4.7, and the 0.63% SnF2 rinses have a pH of 2.8 to 3.5. Because xerostomic patients lack the buffering capacity of saliva, the acidity of the SnF2 products may etch teeth, composite restorations, and porcelain restorations, as well as irritate gingival tissues. The 1.1% neutral NaF products (5,000 ppm) have a more neutral pH, do not etch teeth or dental materials, and are not irritating to the mucosa.2 While long-term studies have shown that the use of these neutral NaF gels reduces the incidence of caries, compliance is a common problem with the use of trays or brush-on techniques.15 One recommendation is to have the patient use a 1.1% neutral NaF (5,000 ppm) dentifrice.15
In addition to the daily use of the neutral 1.1% NaF gels and dentifrices (5,000 ppm), fluoride varnishes such as Duraphat (Colgate Oral Pharmaceuticals) can be used. These contain 5% NaF (22,600 ppm) and are intended for office use.12 They are effective in preventing carious lesions on smooth surfaces.16 The fluoride varnish is painted on the teeth and can be applied every 2 to 3 months. It also is very effective in reducing tooth and root sensitivity.
Radiation-induced xero-stomia will result in an oral environment that promotes the overgrowth of Candida albicans. Patients are likely to require treatment with antifungal medications in addition to the therapies prescribed for dry mouth. The same regimen of antifungal medications described during cancer therapy may be used in the post-cancer therapy stage.3
The post-radiation therapy patient should be placed on a 3-month dental prophylaxis schedule. Good oral hygiene and patient education becomes a lifelong commitment. Note that the exposure to radiation may cause some patients to develop trismus resulting from fibrosis of the mastication muscles. During the post-radiation period, these patients can be placed on a daily regimen of opening and closing exercises using tongue depressors taped together.6 Muscle relaxers such as cyclobenzaprine (Flexeril, McNeil) and antianxiety medications such as diazepam (Valium, Roche Pharmaceuticals) may also be useful in helping to treat trismus.
For the patient requiring fabrication of a removable prosthesis, a thorough evaluation of salivary function and load-bearing tissues should be performed. The limited blood supply to the hard and soft tissues of the mandible may mean that the patient will not be able to tolerate a removable prosthesis. Implants can be considered as an option 12 to 18 months following radiation therapy. If possible, an implant-supported prosthesis is preferable to a conventional denture.2
Osteoradionecrosis is one of the most severe and serious complications of radiation therapy. It involves a nonhealing wound caused by trauma or spontaneous mucosal breakdown exposing hypovascular bone. Patients complain of pain and foul odor, which is due to the accumulation of debris in the defect and exposure of bone to oral fluids. The incidence of osteoradionecrosis is greater for the mandible than for the maxilla, likely because of the greater vascularity of the maxilla.7
Periodontal therapy for irradiated patients should be approached with caution. Flap surgery with or without osseous recontouring should be avoided due to the potential for osteoradionecrosis. Special care should be taken to avoid tissue trauma when providing deep scaling and root planing. If extractions must be performed during the post-radiation period, then primary closure and perioperative antibiotics should be employed. If extensive surgery is required, prophylactic hyperbaric oxygen therapy with antibiotics is advisable.2
CONCLUSION
The dental profession is primarily responsible for the management of oral care for patients undergoing cancer treatment. The dentist should play a key role in early recognition and care as well as in educating the oncologist about the timeliness of such care. Patients should understand that intervention by the dentist could prevent potentially devastating consequences. A long-term partnership between the patient and dentist is paramount in maintaining excellent oral hygiene over a lifetime. The result will be a much-improved quality of life for these patients.
References
1. National Institute of Dental and Craniofacial Research. Oral Complications of Cancer Treatment: What the Oral Health Team Can Do. June 2002. Available at: http://www.nohic.nidcr.nih.gov/campaign/den_fact.htm. Accessed on June 14, 2005.
2. Oral Health in Cancer Therapy: A Guide for Health Care Professionals. 1st ed. Dallas, Tex: Dental Oncology Education Program, Oral Health Education Foundation, and Texas Cancer Council; February, 1999:1-36.
3. Siegel M, Silverman S, Sollecito T, eds. Clinicians Guide to Treatment of Common Oral Conditions. American Academy of Oral Medicine. Ontario, Canada: BC Decker Company; January, 2001:4-19.
4. Joyston-Bechal S. Prevention of dental disease following radiotherapy and chemotherapy. Int Dent J. 1992;42:47-53.
5. Guggenheimer J, Moore PA. Xerostomia: etiology, recognition and treatment. J Am Dent Assoc. 2003;134:61-69.
6. Zlotolow IM. Clinical manifestations of head and neck irradiation. Compend Contin Educ Dent. 1997;18(2 Spec No):51-56.
7. Radiation therapy. AAOMS Surgical Update. (March) 2000;15:2-7.
8. Nguyen AM. Dental management of patients who receive chemo- and radiation therapy. Gen Dent. 1992 Jul-Aug;40(4):305-11.
9. Rothstein JP. Cancer Chemotherapy and Oral Care. Dent Today. 2004;23:86-91.
10. Weikel DS. Oral care for a woman under treatment for breast cancer. Periodontal Management. 1995;1(1):1-8.
11. Jacobsen PL. The Little Dental Drug Booklet. Handbook of Commonly Used Dental Medications. San Francisco, Calif: University of Pacific; 2001-2002 (edition):35-37.
12 Winston AE, Bhaskar SN. Caries prevention in the 21st century. J Am Dent Assoc. 1998;129:1579-1587.
13. Anderson MH. Current concepts of dental caries and its prevention. Operative Dentistry. In: Supplement No. 6, Management Alternatives for the Carious Lesion (Supplement to Operative Dentistry. 2001, Vol. 26). 2001:11-18.
14. Ghalichebaf M, DeBiase CB, Stookey GK. A new technique for the fabrication of fluoride carriers in patients receiving radiotherapy to the head and neck. Compendium. 1994;15:470,473-474,476.
15. DePaola PF. The benefits of high-potency fluoride dentifrices. Compend Contin Educ Dent. 1997;18(Spec No. 2):44-50.
16. Anderson MH, Bales DJ, Omnell KA. Modern management of dental caries: the cutting edge is not the dental bur. J Am Dent Assoc. 1993;124:36-44.
Dr. Barry is the associate dean for clinical affairs in the College of Dental Medicine, Medical University of South Carolina. He is an academic fellow of the American Academy of Oral Medicine and fellow of the American College of Dentists. He has served as a curriculum consultant to the American Dental Association Commission on Accreditation. Dr. Barry can be reached at (843) 792-2142 or barrym@musc.edu.