Throughout the course of our professional lives, we have migrated towards certain habits in our decision making that have become predictive and ingrained. Our decision to employ a particular method or product is often the result of habitual thinking supported by our level of comfort, patterns of use, and previous clinical judgment. Creating habits is relatively simple.
As dental professionals, we are challenged to employ evidence-based decision making. Evidence-based decision making is described as “the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of an individual patient.”1 The process involves the integration of clinical expertise, patient values, and best scientific evidence into our decision making. A scrutiny of literature substantiating evidence-based decision making often challenges our habitual clinical choices—not to mention the time and dedication required to perform a comprehensive literature review.
Fluoride has been widely accepted and integrated as a preventive agent, addressing both the demineralization process that leads to caries development, as well the positive inroads it has made in addressing dentinal hypersensitivity. With the broad range of products that are available to our professional community, evidence supporting the selection of a professionally applied fluoride has become quite complex.
Again, we are often reliant on an ingrained habit rather than adhering to evidence-based and scientifically-supported decision making. The American Dental Association (ADA) Council on Scientific Affairs assembled an expert panel in 2005 to evaluate the collective body of scientific evidence as it related to the efficacy of professionally applied topical fluoride for caries prevention.2 The recommendations were published as a guide, rather than a requirement or regulatory statement, to assist the dental professional in the selection of an effective product.
MedLine and the Cochrane Database of Systematic Reviews were both consulted for clinical studies and systematic reviews of professionally applied topical fluoride including gels, foams, and varnishes. Collective evidence was subsequently evaluated by the selected panel, based on their expertise in relevant subject matter, followed by the release of a detailed document outlining their recommendations entitled, “Professionally Applied Topical Fluoride: Evidence-Based Clinical Recommendations.”2 This document was then submitted for review to scientists with expertise in caries and fluoride efficacy, to ADA agencies, and to 46 organizations representative of academia, professional organizations, industry, and third-party payers. The evidence was further graded and classified according to the strength of the recommendations with (Ia) being the highest category of evidence to (IV) in descending order (Table).
Acidulated phosphate fluoride (APF) gels or foams have a pH of 3.0 to 4.0 which allow for a rapid uptake of fluoride into the enamel especially in the first minute of application. The addition of the phosphate ion was done to counterbalance the effects of the acidic environment and a resulting loss of calcium. Recognition of this rapid uptake in the first minute has led to the recommendation that the clinician may opt to reduce APF treatment time to one minute. Furthermore, in vivo studies are recommended before the ADA would support this protocol as an evidence-based management guideline.
New innovations in fluoride varnish have prompted a movement to shift from in-office use of 1.23% APF gels and foams to 5% sodium fluoride (NaF) varnish. One of the most compelling rationales is the prolonged contact time that fluoride varnish provides. In conclusion, there was clear evidence supporting the efficacy of fluoride varnish for preventing caries in children and adolescents.
THE FLUORIDE MECHANISM
Fluoride is an essential nutrient to the formation of strong teeth and bone structure just like calcium, phosphorus, and other nutritional elements obtained from food and water; it has been recognized as a proven mechanism directly involved in the remineralization of the enamel structure. Before selecting a particular fluoride, it is important to understand the mechanism of fluoride. The mode of delivery for fluoride may be accomplished in 2 ways: systemically by means of introduction into the circulatory system to developing dentition, or, topically by being directly applied to the erupted dentition throughout the lifespan as indicated.
The systemic mechanism for fluoride absorption is initially through intake via the gastrointestinal tract, followed by absorption into the blood-stream. Peak blood levels are reached within 30 minutes. The nutrient is then distributed by plasma to all tissues and organs and then it is naturally drawn to calcified tissues. Approximately 99% of fluoride in the body is located in the mineralized tissues. The fluoride ion is stored in the crystalline structure of teeth and bones. The amount stored is subject to a number of variables including the intake, the time of exposure, age, and stage of development. Highest levels within the tooth structure itself are on the surface of the tooth. Excretion is performed through the urine, with incremental amounts being excreted in the feces and by the sweat glands.
Fluoride occurs naturally, or by means of fluoridation in the water supply. It is also available through a prescribed supplement and in smaller amounts in dietary sources such as meat, eggs, vegetables, cereals, and fruits. Fish will often contain larger amounts of fluoride. Foods prepared in fluoridated water will retain the fluoride gained from the cooking water, as will beverages made from fluoridated water sources. The increase in consumption of bottled water which does not contain optimal fluoride levels (unless stated on the label) is having a negative impact on caries control. Several bottled water manufacturers have included fluoride in their consumer products and are now allowed to claim that “Drinking fluoridated water may reduce the risk of [dental caries or tooth decay].”3 “Widespread use of fluoride has been a major factor in the decline in the prevalence and severity of dental caries (ie, tooth decay) in the United States and other economically developed countries. When used appropriately, fluoride is both safe and effective in preventing and controlling dental caries.”3
 |
 |
|
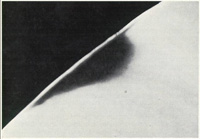
Figure 1. Mineral-rich outer enamel layer with subsurface demineralization. |
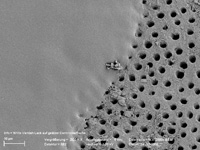
Figure 2. Occlusion of dentin tubules (on left) following application of 5% NaF varnish. |
Fluoride is a natural constituent of the enamel with the outer surface containing the highest concentration due to it being closest to the source of fluoride. Concentrations of fluoride in biofilm and saliva inhibit the demineralization of sound enamel and enhance the remineralization/recovery of demineralized enamel. A certain amount of mineral ions can be lost without the enamel losing its structural integrity (Figure 1). As cariogenic bacteria metabolize carbohydrates and produce acid, fluoride is released. The released fluoride is combined with calcium and phosphate to establish an improved enamel crystalline structure. Fluoride is more readily taken up by the demineralized enamel than by sound intact enamel. Ongoing cycles of demineralization and remineralization continue throughout the lifespan of the dentition with topical application being the most effective means of inhibiting caries development.
FLUORIDE SELECTION FOR MANAGEMENT OF DENTINAL HYPERSENSITIVITY
With today’s dental patient retaining their dentition for a much longer period of time, dental professionals are challenged to assisting the patient with the maintenance of a comfortable dentition.
Dentinal hypersensitivity may be defined as pain arising from exposed dentin, typically in response to chemical, thermal, or osmotic stimuli that cannot be explained as arising from any other form of dental defect or pathology.4 The most widely accepted theory is Brann-strom’s (hydrodynamic) theory suggesting that various stimuli cause fluid to move within the dentinal tubules which in turn excites the nerve endings in the pulp. Scanning electron microscopy reveals that patients who experience dentin hypersensitivity have a greater number of tubules with a diameter greater than in patients with no sensitivity. This variance in size will contribute to a greater fluid flow.
Published literature estimates that between 20% to 45% of the population has experienced dentin hypersensitivity. Many adults report that sensitivity is intermittent, resulting in a suggested clinical prevalence that may underestimate the actual incidence. More recent investigation and documented studies attest to the fact that hypersensitivity is more prevalent than was first thought.
A large scale survey was conducted among a representative sample of the US population to look at prevalence of tooth sensitivity; as reported by participants along with a history of sensitivity toothpaste usage and attitudes. The survey was completed by a gender and regional balanced group of 1,056 volunteers. Only 18% of respondents reported having never had sensitivity (thus 82% had some experience with sensitivity and roughly one half reported experiencing it at least occasionally if not more frequently).4
A number of chairside and at-home products have been proposed for treatment of dentinal hypersensitivity. Fluoride varnish applied in office is an ideal choice due to its prolonged contact time with the tooth structure. Calcium fluoride, and to some degree fluorapatite, may be laid down on the dentinal surface aiding in desensitization. As illustrated in Figure 2, fluoride varnish has the ability to occlude the tubules.
EVIDENCE-BASED GUIDELINES FOR FLUORIDE SELECTION
In vitro and in vivo fluoride uptake, from both a perspective of reaction and release in the enamel, are strongly influenced by the length of contact with the fluoride agent. The availability of the fluoride in the liquid phase around the apatite crystallites is currently believed to be more important in decreasing dissolution of crystallites than fluoride incorporation in the crystal lattice.5 Fluoride varnishes may be optimal in this respect. Varnishes prolong contact time between the tooth and the fluoride compared with other delivery methods. It appears that the main cariostatic effect of fluoride varnish is caused by the remineralization of early carious lesions.
In comparison to gel and foam, fluoride varnish has been found to not only be effective but also may be a preferred method. This is due to the lower amount of time required in its application, as well as a more controlled fluoride exposure.
An evaluation of the results of the available in vitro animal and human studies on the anticaries efficacy of these preventive agents was performed. The results of the meta-analysis were that the efficacy of fluoride varnishes in caries prevention is clearly demonstrated in several experimental studies. Clinical trials show caries incidence reduction of approximately 70%.
A study was conducted on 1,251 children, ages 6 to 12 years, in an effort to evaluate the effect of topical fluoride application. NaF, APF, and Duraphat (NaF varnish) were given at 6-month intervals and assessed after 2.5 years. The study revealed the percentage of caries reduction (with NaF to be in the range of 20% to 24% on base line teeth and 30% to 33% on teeth erupted during the study) showing more effect on newly erupted teeth. In the APF group, the caries reduction was 32% to 37%. The dental caries reduction with the NaF varnish was in the range of 70% to 75%—slightly more on newly erupted teeth. Equally high degree of efficacy was also noted on occlusal surfaces. Duraphat showed the greatest public health potential.6
| Table. Statements Released and Approved by the ADA Council on Scientific Affairs Regarding Fluoride Administration. |
|
A number of other scientific literature reviews regarding the use of fluoride therapies in prevention of dental caries have been published since the year 2000, including 2 evidence-based reports.7-9 The Cochrane reviews of this topic concluded that “Fluoride varnishes applied professionally, 2 to 4 times a year, would substantially reduce tooth decay in children.8
CLINICAL CONSIDERATIONS
Several articles have addressed the potential for variance in fluoride gradients to occur within multidose varnish vials.10-12 The fluctuation in gradients are suggested to be the result of separation of the fluoride in multidose preparations. It is through these observations that the recommendation has been made to use a single-dose preparation, stirring it well prior to application. A systematic review, with an objective to develop a scientifically current and evidence-based protocol for the use of fluoride varnish for the prevention of dental caries among high-risk children and adolescents, conducted by Dr. Azarpazhooh13, Community Dental Health Research Unit, Faculty of Dentistry, University of Toronto provided the following statement, “Consistent availability of fluoride in the varnish preparation is very important to efficacy and cannot be assured with multidose packages.”13
CavityShield varnish (OMNI Preventive Care, a 3M ESPE Company), the first unit-dosed 5% neutral NaF varnish, is available in bubblegum flavor and 2 convenient sizes. The unit-dose feature provides the ability to mix fluoride varnish (which naturally settles) prior to application thus offering assurance of consistency, concentration, and safety. Studies have revealed that there is a more uniform fluoride content in Cavity-Shield unit-dose packages than in traditional NaF varnish tubes. If varnish is dispensed from a tube (rather than a single-dose packet), discard any clear varnish because the ingredients have separated and will contain only a fraction of the intended amount of fluoride.12
 |
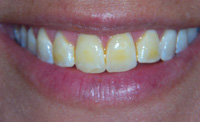 |
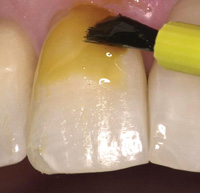
| Figure 3. Traditional appearance of 5% NaF Varnish. |
Figure 4. Traditional 5% NaF varnish. |
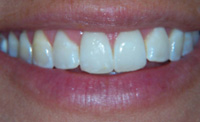 |
| Figure 5. Appearance of dentition following 5% NaF application of VANISH (OMNI Preventive Care—a 3M ESPE company). |
OMNI Preventive Care has addressed the consumer response and unveiled a product called Vanish (Figure 5). Vanish virtually disappears after applying in a thin layer to the dentition, so there is no trace of the unsightly yellowish/brown color that was evident with traditional varnishes.15
CONCLUSION
It is clear that some of the recommendations that emerged following the comprehensive review of supporting literature have challenged our habitual selection of professionally applied fluorides. This knowledge is empowering and will further guide us into treatment decisions that will offer the highest degree of efficacy given a patient’s health and dental history, vulnerability to oral disease, and the overall best interests of each individual patient.
With product availability meeting consumer demands and clear evidence supporting the efficacy of fluoride varnish, we are now in a position as clinicians to challenge our traditional selection of fluoride application. Let the evidence be your guide.
References
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
- American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc. 2006;137:1151-1159.
- CDC. Recommendations for Using Fluoride to Prevent and Control Dental Caries in the United States. MMWR Recomm Rep. 2001;50(RR-14):1-59.
- General population tooth sensitivity prevalence and attitudes towards sensitivity toothpaste. NovaMin Research Report. Greenfield Market Research, 2004. http://www.novamin.com/pdf /research_reports/General_Population_Tooth. pdf. Accessed January 15, 2009.
- Arends J, ten Cate JM. Tooth enamel remineralization. J Crystal Growth. 1981;53:135-147.
- Beltran-Aguilar ED, Goldstein JW, Lockwood SA. Fluoride varnishes. A review of their clinical use, cariostatic mechanism, efficacy and safety. J Am Dent Assoc. 2000;131:589-596.
- Hawkins RJ, Locker D. Evidence-based recommendations for the use of professionally applied topical fluorides in Ontario’s public health dental programs. Toronto, ON, Canada: University of Toronto, Faculty of Dentistry, Community Dental Health Services Research Unit; 2001. Quality Assurance Report no. 20. http://www.caphd-acsdp.org/PDF/ebd-fluo.pdf. Accessed January 17, 2009.
- Marinho VC, Higgins JP, Logan S, et al. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2002;(3):CD002279.
- Marinho VC, Higgins JP, Sheiham A, et al. One topical fluoride (toothpastes, or mouthrinses, or gels, or varnishes) versus another for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2004;(1): CD002780.
- Castillo JL, Milgrom P. Fluoride release from varnishes in two in vitro protocols. J Am Dent Assoc. 2004;135:1696-1699.
- Eakle WS, Featherstone JD, Weintraub JA, et al. Salivary fluoride levels following application of fluoride varnish or fluoride rinse. Community Dent Oral Epidemiol. 2004;32:462-469.
- Shen C, Autio-Gold J. Assessing fluoride concentration uniformity and fluoride release from three varnishes. J Am Dent Assoc. 2002;133:176-182.
- Azarpazhooh A, Main PA. Fluoride varnish in the prevention of dental caries in children and adolescents: a systematic review. J Can Dent Assoc. 2008;74:73-79,79a-79j.
- Wilkins EM. Clinical Practice of the Dental Hygienist. 9th ed. Hagerstown, Md: Lippincott Williams & Wilkins; 2004:555.
- Vanish 5% NaF White Varnish. Omni Preventive Care Web site. http://solutions.3m.com/wps/portal/3M/en_US/preventive-care/home/products /in-office-therapies/vanish. Accessed January 15, 2009.
Ms. Jones is an international lecturer, consultant, author, and practicing clinician. Working closely with the corporate sector, she remains active as a key opinion leader and professional educator focusing on best clinical practices and the enjoyment of best treatment outcomes. She may be contacted at jjones@rdhconnection.com.
Disclosure: Ms. Jones is involved in professional development programs offered by 3M ESPE.