Dentists perform a variety of surgical procedures frequently requiring the need for a hemostatic agent. Exodontia, tissue biopsies, placement of endosseous implants, and periodontal surgery are just some examples where hemostatic agents may be beneficial. Not only are these agents useful for specific procedures, but they also are valuable for certain patient groups, specifically those with coagulation defects. These defects may be genetic or acquired (Table). A comprehensive review of this topic is beyond the scope of this article. Nevertheless, dentists performing surgery should be familiar with these defects and their clinical manifestations. Figure 1 demonstrates a schematic diagram of the cascade of events leading to the formation of a fibrin clot. These steps are initially divided into intrinsic and extrinsic pathways, leading into a common pathway of coagulation.
This article is a review of perioperative hemorrhage, certain available hemostatic agents, and an introduction to a new agent with good potential for application in the oral cavity.
PERIOPERATIVE HEMORRHAGE
The best management of perioperative hemorrhage is prevention. This includes a thorough preoperative patient history, necessary medical consults, familiarity with managing patients with possible bleeding diathesis, meticulous intraoperative technique, and appropriate postoperative instructions, care, and follow-up. Although these are easily listed, application in practice can be challenging. Multiple obstacles may prevent the implementation of the management steps listed. Some of these hurdles include treating patients with an undisclosed or undiagnosed medical condition, improper information retrieval, or difficult surgical conditions. Poor patient compliance with medication or postoperative instructions also are factors to be considered.
If an intraoperative bleeding episode is encountered, the clinician should consider several steps. A quick mental review of the patient’s medical history is first. If the hemorrhagic episode is difficult to manage, injection of 1/50,000 solution of epinephrine into the area may be needed. This will likely provide temporary reduction of bleeding as a result of local vasoconstriction. The site may need to be packed, and the clinician will need to consider the seriousness of the event. If very serious, a call to an emergency service (911) may be necessary. Further, if the dentist is properly trained, starting an intravenous line to initiate fluid resuscitation may be advisable. The process of immediate delivery of the patient to a medical facility for possible transfusions, anticoagulant reversal, and general life support measures can be initiated. However, a careful clinician will rarely encounter such an event in an outpatient office setting. More commonly, dentists confront patients with inconvenient, nonemergent bleeding events that require a response.
During oral surgical procedures, persistent minor oozing of blood is common, although occasionally a bleeding episode prevents the continuation of the procedure and requires immediate attention. The usual sources for this intraoperative complication are incision into an area of granulomatous tissue, vessels in the periosteum or mucosa, or encountering nutrient arteries in the alveolar bone. Identification of the source of the bleeding requires good illumination, adequate retraction, and thorough suctioning. Once identified, the bleeding site should be packed, clamped, cauterized, burnished, debrided, and/or sutured for control. Topical hemostatic agents should be available, and if necessary, applied.
The dentist should be familiar with the range of methods, techniques, materials, and their application during different types of bleeding episodes. Having a broad knowledge of the management approaches will allow the clinician to know when to apply a particular approach. Unfortunately, some of the most useful preventive measures and management techniques are not utilized because of a lack of understanding of the coagulation process and/or the approaches and materials that are available.
One of the more common methods of intraoperative hemorrhage control involves the use of a topical hemostatic agent. This article reviews some of the more common hemostatic agents and introduces ActCel (MedSpring Group), a hemostatic agent that has recently become available.
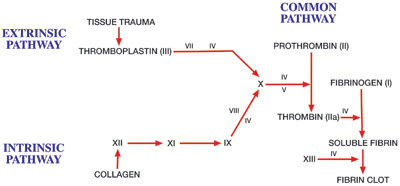 |
| Figure 1. Coagulation cascade. (Reprinted from Handbook for Anesthesia and Co-Existing Disease, Vol 1, Robert K. Stoelting, MD, and Stephen F. Dierdorf, MD, page 256, 1993, with permission from Elsevier.) |
 |
| Figures 2a and 2b. Left: A 1 x 1-inch piece of hemostatic gauze. Right: The same piece of gauze that has been placed for a few seconds into water. Upon saturation with water or blood, the gauze starts to convert to a resorbable, gel-like state. In a fresh extraction socket, it serves to promote hemostasis and stabilize a blood clot. |
 |
 |
| Figure 3. The cotton plier is grasping a 1 x 1-inch piece of hemostatic gauze. It is ready to be placed in a lower third molar socket. The purpose of the gauze is to help stabilize the clot and prevent a dry socket. It has been lightly coated with tetracycline. (Approximately 20 1 x 1-inch pieces of the gauze were place in a sterile container to which the contents of one 250-mg capsule of tetracycline was added. The container was then shaken to coat each piece uniformly with a small amount of tetracycline powder.) As the gauze enters the socket, it is easily controlled and manipulated with the cotton pliers. | Figure 4. The gauze is placed in and out of the socket until it is totally saturated with blood. When saturated, it can be left in the socket, since it readily converts to glucose and saline within 1 to 2 weeks. One or more sutures can then be placed superiorly to close the wound. |
 |
 |
| Figure 5. Preoperative radiograph of tooth No. 32—a partial bony impaction. The patient is a 32-year-old male and is healthy with no known bleeding problems. | Figure 6. In the process of removing bone with a bur on the disto-buccal aspect of this tooth (see x-ray in Figure 5), a nutrient canal (artery) was cut. Some spurting of blood ensued. The vessel was localized by the use of suction, and bone surrounding the bleeding source was compressed into the site with a periosteal elevator. Bleeding lessened but still continued. The next step was to burnish Bone Wax into the bleeding bony orifice. Again, the magnitude of bleeding diminished but did not stop. Note the amount of blood collected on 4 x 4-inch gauze sponges during this period of time. |
 |
 |
| Figure 7. Since bleeding was not yet completely controlled (continued from Figures 5 and 6), 1 x 1-inch pieces of hemostatic gauze were placed in the socket one-by-one until the bleeding totally stopped. A total of 8 pieces were placed. After a few minutes, 3 sutures were tied to approximate soft tissue over the socket. This helped contain the gauze and protect it from dislodgement. After 30 minutes of observation, the patient was allowed to leave the office. | Figure 8. The immediate postextraction socket of tooth No. 5 in an 82-year-old woman who was taking Coumadin. She had an INR of 3.0. Preoperatively, her physician was consulted over the phone. It was decided that with her stroke history and the limited nature of the oral surgery, it would be best to use local measures to control expected intraoperative and possible postoperative hemorrhage. At the surgery appointment, the tooth was removed atraumatically. Following the extraction, one 1 x 1-inch piece of hemostatic gauze was placed in the socket. A figure-8 suture was tied over the socket to better approximate buccal and lingual gingiva and to prevent subsequent hemostatic gauze dislodgement. As a precaution, food was limited to a liquid diet that day. Food was chewed on the other side of the mouth for the following few days. |
TOPICAL HEMOSTATIC AGENTS
Hemostatic Collagen
These products (eg, CollaPlug, CollaTape, and Helistat [Integra LifeSciences]) are soft, white, pliable, nonfriable, coherent, sponge-like structures. They are fabricated from bovine collagen (usually from deep flexor tendons) and are nontoxic and nonpyrogenic. The products are highly absorbent and able to hold many times their own weight of fluid. Their indications are for wound protection and for control of oozing or bleeding from clean oral wounds. As for application, these products should be held in place for approximately 2 to 5 minutes to achieve hemostasis and then may be removed, replaced, or left in situ. All of these collagen materials are completely resorbed within 14 to 56 days.1
In addition to serving as a mechanical obstruction to bleeding, these materials affect the coagulation process. In contact with blood, collagen causes aggregation of platelets, which bind in large numbers to the collagen fibrils. The aggregated platelets degranulate, releasing factors such as thromboxane A22 that assist in the formation of a clot. The sponge also provides a 3-D matrix for strengthening the blood clot.3
As with most hemostatic agents, collagens are not to be used in infected or contaminated wounds. The agents may serve as a nidus for abscess formation and may potentiate bacterial growth. Possible adverse reactions are formation of adhesions, allergic reactions, foreign body reactions, and subgaleal seroma formation3 (subgaleal seroma is an accumulation of blood serum beneath the scalp). In an animal model, incision sites inoculated with Staphylococcus aureus demonstrated more infection when collagen was used as compared to a control.3 Such results are similar to what has been reported for other hemostatic agents.3
Besides sponges and plugs, these products are available in microfibrillar form. This form is generally less useful for oral surgical procedures.
|
Table. Categories of Hereditary hemophilia A Acquired vitamin K deficiency |
Gelatin
(eg, Gelfoam)
Gelfoam (Pharmacia) is one of the more commonly employed agents for the control of minor bleeding. It is a porous, pliable sponge made from dried and sterilized porcine skin gelatin. Gelfoam’s mode of action is not completely understood, but unlike collagen, it is believed to be related to formation of a mechanical matrix that facilitates clotting4-7 rather than affecting the blood-clotting mechanism. This agent can retain in its interstices 45 times its weight in blood.8 Gelfoam liquefies in one week and is completely resorbed in 4 to 6 weeks.
Its use is not associated with excessive scar formation.8-12 Reported adverse reactions are giant cell granuloma and hematoma formation, foreign body reactions, excessive fibrosis, toxic shock syndrome, fever, and failure of absorption.13
Bone Wax
Bone Wax (Ethicon) is a sterile mixture of beeswax, paraffin, and isopropyl palmitate (a softening agent) that is packaged in individual foil envelopes. It is useful when bleeding is from a visualized local vascular channel within bone, commonly referred to as a “bone bleeder,” at the surgical site. This occurs commonly during the extraction of mandibular third molars, and if not adequately addressed during surgery can be a reason for postoperative bleeding. The wax is pliable enough to be placed within a vascular channel, immediately tamponading the vascular source.
Bone Wax is nonresorbable, and due to its possible adverse effect on osteogenesis,14 caution should be used where regeneration of bone is expected (eg, a future implant site). Mild inflammatory reactions have been reported in tissues adjacent to the site of Bone Wax implantation,14 and this agent may prevent the clearing of bacteria from infected sites.1
Cellulose (eg, Surgicel, ActCel)
Surgicel (Johnson & Johnson) is a resorbable oxidized cellulose material and is an expensive but useful option in oral surgery. It is prepared as a sterile fabric meshwork. Its mechanism of action is not completely understood, but appears to be physical rather than involve an alteration of the clotting mechanism. After it is fully absorbed with blood, it swells into a brownish/black gelatinous mass that aids in clotting. Excessive amounts of the material should be removed if possible to prevent delayed healing.
Specific dental indications include use as an adjunct to control bleeding in exodontia and other oral surgical procedures.15 This material may be more useful in soft-tissue procedures due to its shape, consistency, and interference with osteogenesis.
Surgicel has been found to be bacteriocidal in vitro against many organisms, and it does not enhance infection under experimental conditions.16-19 Nevertheless, it is still recommended to avoid its use in contaminated wounds where persistent drainage is desired. Encapsulation of fluid and foreign body reactions have been reported,20-22 and a burning sensation has been noted when the product is placed in unanesthetized nasal passages.15
ActCel is a new topical hemostatic agent that is made from treated and sterilized cellulose and available in similar fabric meshwork as Surgicel, although it is slightly more friable. It is an FDA-approved material indicated for the control of bleeding from open wounds and body cavities (eg, mouth, ears, nose, throat, and vagina). The material does not contain chemical additives, thrombin, or collagen, and is hypoallergenic. In contact with blood, it expands to 3 to 4 times its original size and is almost immediately converted to a gel. Complete dissolution of the product takes place within 1 to 2 weeks.23 Because of its purity and the fact that it degrades rapidly into biocompatible end products (glucose, water), it does not adversely affect wound healing. ActCel’s mechanisms of action are multiple, enhancing the coagulation process biochemically by en-hancing platelet aggregation and physically by 3-D clot stabilization (Figure 2).
After an injury, platelets are activated, changing from a discoid to spherical shape and extending pseudopodia. Platelets release factors such as thromboxane A22 that stimulate other platelets to activate. Platelets have receptors that promote their adhesion to blood vessel linings, collagen, and other platelets.2 It has been demonstrated that the fabric-like and solid texture of ActCel slows blood flow and reduces the time for thrombin to be released into the wound.24 This increases platelet adhesion (similar to platelet aggregation at a damaged blood vessel wall or at exposed collagen), thereby reducing the time to establish the clot.
Subsequently, coagulation progresses through a cascade of events with the relatively unstable platelet plug being replaced with a stronger, more resilient clot. This cascade involves a series of interdependent, enzyme-mediated reactions that are initiated by the degranulation of platelets or the release of activated tissue thromboplastin from damaged blood vessels. The fundamental reaction is the generation of thrombin and fibrin from prothrombin and fibrinogen, respectively. Clinically, the blood tests performed to detect abnormalities within this series of events are partial thromboplastin time (PTT) to assess the intrinsic pathway or prothrombin time (PT) and international normalized ratio (INR) to evaluate the extrinsic pathway. These tests measure different phases of early aspects of the coagulation process (intrinsic and extrinsic pathways), but all measure the common pathway and formation of end products.25 Research has demonstrated that in vitro, PTT is decreased (resulting in en-hanced clotting) with the presence of ActCel in the wound.24 (The procedure consists of placing defined weight, or a surface area equivalent, of test material in contact with citrated plasma, incubation, and then adding a PTT reagent and calcium chloride. Time required for clotting is then determined for the test sample and the control, which has no device contact. A minimum of 6 tests were performed, averaged, and statistically analyzed using analysis of variance.) These data support the role of ActCel in modifying the intrinsic pathway.
Additionally, many of the previously mentioned enzyme-mediated reactions within the clotting cascade are calcium dependent. One study has demonstrated that ActCel adheres to calcium ions,23 making calcium more available for the clotting cascade. Further, since the material’s surface area increases as it dissolves, the area available for the coagulation process also increases.
Another special characteristic of this material is its bacteriostatic properties.24 This is especially important in contaminated wounds or in body cavities in which it is difficult or impossible to maintain a sterile field.
Dry Socket Prevention
Localized alveolar osteitis is the most common complication in extractions, with a prevalence of 1.9% to 31.2% following removal of mandibular third molar teeth.26-28 A variety of theories exist as to its etiology, although it certainly involves an interruption of the healing process. This condition is a significant problem for both the clinician and the patient, as 45% of patients who develop alveolar osteitis require at least 4 additional postoperative visits.29 Several methods have been found to reduce the incidence of alveolar osteitis, including the use of both topical30-32 and systemic agents.33-35 Preliminary results examining the use of ActCel and tetracycline in mandibular impacted extraction sites have reduced alveolar osteitis (personal observation). Randomized controlled trials are needed to substantiate these observations.
Indications for the use of ActCel are the control of bleeding or protection of a wound in the oral cavity. This includes minor oozing that may require additional hemostatic assistance, routine use in mandibular third molar extraction sites to decrease the occurrence of alveolar osteitis, or as an adjunct for control of arterial bleeding (first stopping the arterial bleed with other methods and then assisting in stabilizing the clot by placement of ActCel). ActCel, as previously noted, should not be placed in areas that are not in communication with the external environment or placed in infected sites (Figures 3 to 8).
CONCLUSION
Hemostatic agents are used in dentistry for hemorrhage control and wound protection. This article has reviewed different hemostatic agents, their mechanisms of action, and their clinical indications and contraindications. ActCel, a new hemostatic agent, was reviewed.
References
- Ogle OE. Perioperative hemorrhage. In: Dym H, Ogle OE. Atlas of Minor Oral Surgery. Philadelphia, Pa: WB Saunders; 2000:62-63.
- Platelets and primary hemostasis. In: Andreoli TE, Bennett JC, Carpenter CCJ, et al. Cecil Essentials of Medicine. 4th ed. Philadelphia, Pa: WB Saunders; 1997:403.
- CollaPlug [package insert]. Plainsboro, NJ: Integra LifeSciences Corp; 2001.
- Guralnick W, Berg L. GELFOAM in oral surgery. Oral Surg. 1948;1:629-632.
- Jenkins HP, Janda R, Clarke J. Clinical and experimental observations on the use of gelatin sponge or foam. Surgery. 1946;20:124-132.
- Jenkins HP, Janda R. Studies on the use of gelatin sponge or foam as a hemostatic agent in experimental liver resections and injuries to large veins. Ann Surg. 1946;124:952-961.
- Correll JT, Prentice HR, Wise EC. Biologic investigations of a new absorbable sponge. Surg Gynecol Obstet. 1945;181:585-589.
- Council on Pharmacy and Chemistry. Absorbable gelatin sponge: new and nonofficial remedies. JAMA. 1947;135:921.
- Jenkins HP, Senz EH, Owen H, et al. Present status of gelatin sponge for control of hemorrhage. JAMA. 1946;132:614-619.
- Treves N. Prophylaxis of post mammectomy lymphedema by the use of GELFOAM laminated rolls. Cancer. 1952;5:73-83.
- Barnes AC. The use of gelatin foam sponges in obstetrics and gynecology. Am J Obstet Gynecol. 1963;86:105-107.
- Rarig HR. Successful use of gelatin foam sponge in surgical restoration of fertility. Am J Obstet Gynecol. 1963;86:136.
- Gelfoam [package insert]. Kalamazoo, Mich: Pharmacia; 1999.
- ETHICON Bone Wax [package insert]. Somerville, NJ: Ethicon Inc; 1997.
- Surgicel Fibrillar Hemostat [package insert]. Somerville, NJ: Ethicon Inc; 1998.
- Dineen P. Antibacterial activity of oxidized regenerated cellulose. Surg Gynecol Obstet. 1976;142:481-486.
- Dineen P. The effect of oxidized regenerated cellulose on experimental intravascular infection. Surgery. 1977;82:576-579.
- Dineen P. The effect of oxidized regenerated cellulose on experimental infected splenotomies. J Surg Res. 1977;23:114-125.
- Kuchta N, Dineen P. Effects of absorbable hemostats on intraabdominal sepsis. Infections in Surgery. 1983;2:441-445.
- Ibrahim MF, Aps C, Young CP. A foreign body reaction to Surgicel mimicking an abscess following cardiac surgery [letter]. Eur J Cardiothorac Surg. 2002;22:489-490.
- Krishnan LK, Mohanty M, Umashankar PR, et al. Comparative evaluation of absorbable hemostats: advantages of fibrin-based sheets. Biomaterials. 2004;25:5557-5563.
- Kothbauer KF, Jallo GI, Siffert J, et al. Foreign body reaction to hemostatic materials mimicking recurrent brain tumor: report of three cases. J Neurosurg. 2001;95:503-506.
- Zhang Qin-shang, Xu Qing-zhong. Application of S-99 soluble styptic gauze to wounds. Beijing Xuan Wu Hospital, Departments of Pathology and Stomatology. Beijing, China. December 31, 1982: personal communication.
- Data on file. Nelson Laboratories, Inc; Salt Lake City, Utah; Telephone: (801) 963-2600.
- Hematologic problems. In: Barker LR, Burton JR, Zieve PD eds. Principles of Ambulatory Medicine. 5th ed. Baltimore, Md: Williams & Wilkins; 1999:641.
- Berge TI, Boe OE. Predictor evaluation of postoperative morbidity after surgical removal of mandibular third molars. Acta Odontol Scand. 1994;52:162-169.
- Swanson AE. Prevention of dry socket: an overview. Oral Surg Oral Med Oral Pathol. 1990;70:131-136.
- Krekmanov L. Alveolitis after operative removal of third molars in the mandible. Int J Oral Surg. 1981;10:173-179.
- Osborn TP, Frederickson G Jr, Small IA, et al. A prospective study of complications related to mandibular third molar surgery. J Oral Maxillofac Surg. 1985;43:767-769.
- Quinley JF, Royer RQ, Gores RJ. “Dry socket” after mandibular odontectomy and use of soluble tetracycline hydrochloride. Oral Surg Oral Med Oral Pathol. 1960;13:38-42.
- Goldman DR, Kilgore DS, Panzer JD, et al. Prevention of dry socket by local application of lincomycin in Gelfoam. Oral Surg Oral Med Oral Pathol. 1973;35:472-474.
- Julius LL, Hungerford RW, Nelson WJ, et al. Prevention of dry socket with local application of Terra-Cortril in gelfoam. J Oral Maxillofac Surg. 1982;40:285-286.
- Rood JP, Murgatroyd J. Metronidazole in the prevention of ‘dry socket’. Br J Oral Surg. 1979;17:62-70.
- Krekmanov L, Nordenram A. Postoperative complications after surgical removal of mandibular third molars: effects of penicillin V and chlorhexidine. Int J Oral Maxillofac Surg. 1986;15:25-29.
- Mitchell DA. A controlled clinical trial of prophylactic tinidazole for chemoprophylaxis in third molar surgery. Br Dent J. 1986;160:284-286.
Dr. McBee is an oral and maxillofacial surgeon who graduated from dental school at the University of Tennessee and then completed his residency at the University of Minnesota. He currently practices oral and maxillofacial surgery in Provo, Utah, and can be reached at (801) 375-4707 or mcbee0006@msn.com.
Disclosure: Dr. McBee is a member of the MedSpring Group Medical Advisory Board. He does not receive financial remuneration for any product mentioned in this article.
Dr. Koerner has taught more than 500 didactic and hands-on courses to dentists in the United States and abroad. He has contributed to 2 volumes of Dental Clinics of North America. Since 2002, he has been a clinician and lecturer for Clinical Research Associates in Provo, Utah. He is past president of the Utah Dental Association and Utah Academy of General Dentistry. He is a general dentist and practices in Salt Lake City, Utah, and can be reached at (801) 502-8585 or kr-koerner@comcast.net.
Disclosure: Dr. Koerner is a member of the MedSpring Group Medical Advisory Board. He does not receive financial remuneration for any product mentioned in this article.