INTRODUCTION
In the business world, there is the term “job to be done.”1 This concept relates to how a consumer purchases a product or service. A “job” is a problem that a person is trying to solve. In reality, a consumer doesn’t really buy a product or service, but rather they “hire” it to get a “job done.” When it comes to dental care, specifically endodontic treatment, a dentist needs to “hire” specific products (instruments) and techniques (services) that will enable him or her to properly treat his or her patient (ie, the “job to be done”). On the other hand, a patient typically presents to a dentist’s office or clinic with his or her own “job to be done” of “help me eliminate this tooth pain that is hurting so badly that it has kept me up the last few nights.” The patient then “hires” a dentist to provide a treatment (service) to eliminate his or her tooth pain.
The current clinical armamentarium and techniques used in endodontic treatment that we “hire” to prepare root canal systems have significantly improved our patient outcomes (“job to be done”).2 We have seen endodontic files change in shape, taper, and type of metal (ie, stainless steel to NiTi),3 along with one of their most significant changes: evolving from hand to rotary file usage. Also, we have a better understanding that endodontic canal preparation involves a chemomechanical process. The mechanical portion is the use of files, and the chemo portion of the process is the use of medicaments such as ethylenediaminetetraacetic acid (EDTA), chlorhexidine, and calcium hydroxide (Ca[OH]2) in conjunction with sodium hypochlorite (NaOCl).4-6 Advancements in visualization through loupes, microscopes, and/or endoscopes have provided the clinician with a better visual vantage point for canal preparation.7,8 Other advancements in apex locators, radiography, and CBCT have also enabled clinicians to better understand the root canal morphologies they are treating.9,10 Yet with all the current advancements in endodontic canal preparation, we still do not consistently address vital pulp treatment, variations of canal morphology, residual canal debris, maintaining correct working length, and reducing procedural errors during canal obturation. Part of this problem is due to the fact that, in endodontic treatment, the dentist does not directly see all the complicated root anatomy he or she is treating. Instead, he or she needs to rely on 2-D radiographs that represent the 3-D biological canal. However, even with the advent of CBCT usage in endodontics that provides a more 3-D canal image,9 the canal morphology that it reveals still provides a clinical challenge for the clinician in properly chemomechanically preparing a root canal system.
 |
As dentists, we are continually inundated with new rotary NiTi file systems. Endodontic file manufacturers are always trying to appeal to our clinical need for making canal preparation as simple and effective as possible using the least number of files. Unfortunately, these new files all have one flaw: their inability to anatomically prepare canals. Therefore, clinically, files still do not address the basic problem of properly debriding a root canal system.11 The reason for this is that NiTi rotary files stay centered in a canal when rotating and, therefore, do not contact the dentinal walls that have various invaginations or irregularities.12,13 The reason dentists need to be concerned about the residual canal debris left in a canal after preparation is that this organic and inorganic debris has been associated with endodontic treatment failure. In a study by Lin et al,14 it was found that the major factor associated with endodontic failures is the persistence of a bacterial infection in the canal space or periradicular area.
In addition to all the physical morphological challenges we face in properly preparing a canal, there are quantitative challenges too. It is not uncommon for a dentist to struggle during endodontic treatment in regard to determining and maintaining the proper working length throughout the canal preparation and determining the correct apical size to enlarge a canal. This is all completed while not transporting, ledging, or perforating the canal.15
The question then arises: How can we prepare a root canal by addressing a canal’s natural morphology and residual canal debris, eliminating some of the quantitative instrumentation challenges, and reducing procedural error with obturation (overfill) while obtaining better treatment outcomes?
In order to start this process of finding the solution to our current root canal preparation and obturation challenges, we first need to think more like a physician than a dentist! This may seem way outside the box, but in reality, it’s not. If a physician were to perform root canal treatment, he or she would not be caught up in all the quantitative assessments that we as dentists become obsessed about. Physicians would look at it more qualitatively, the way they perform many of the common surgeries (orthopedic surgery, general surgery, plastic surgery, etc). In performing endodontic treatment, a physician would remove the inflamed or infected pulpal tissue while preserving as much of the natural canal morphology as possible. He or she would not be caught up on canal diameter tapers, 0.5-mm measurements, etc. Lastly, he or she would also think about performing endodontic treatment from more of a biological approach.
BIOACTIVE ENDODONTICS
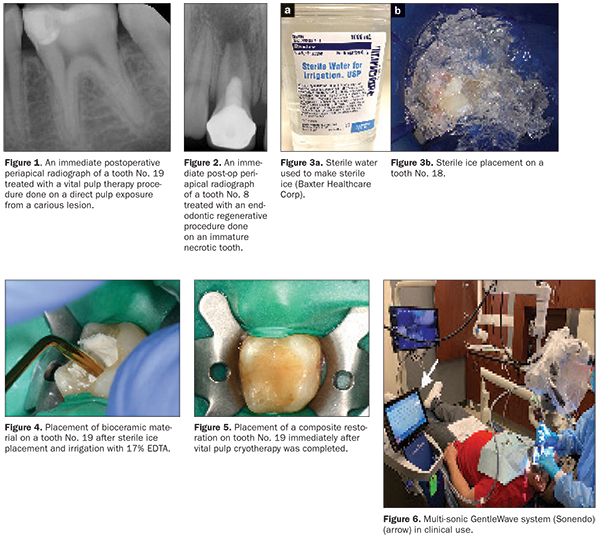
As dentists now thinking as “oral physicians,” we better understand that the future direction of endodontics is bioactive endodontics. By definition, bioactive means having a biological effect. Bioactive endodontic treatment can be achieved via either vital pulp cryotherapy or regenerative endodontics. Vital pulp cryotherapy integrates the use of sterile ice (cryotherapy) in conjunction with EDTA, bioceramics (BCs), and restorative materials on pulp tissue that has been exposed or indirectly exposed due to a carious lesion (Figure 1). Regenerative endodontics has been shown to provide a more biological approach to conventional endodontic treatment than the current clinical methodology. This procedure can be done on both vital and necrotic pulps of immature and mature permanent teeth. Regenerative endodontics uses periradicular blood to fill a prepared canal and thus eliminates the use of warm vertical and cold lateral compaction techniques, along with carrier-based root-filling materials for canal obturation (Figure 2). The tissues generated in the canals after a regenerative endodontic procedure have been shown to be cementum-like, bone-like, and periodontal ligament-like tissues with blood vessels and nerves. Although these tissues are not true pulpal tissue, they are the host’s own vital tissue as opposed to foreign obturation materials (gutta-percha and sealer).16
 |
VITAL PULP CRYOTHERAPY
There has been a paradigm shift in vital pulp therapy over the last 3 to 5 years. Prior to this time, vital pulp treatment has often been viewed as a temporary pulpal procedure, rather than a permanent one.17 When evaluating the “old school” technique for vital pulp therapy, it was done using NaOCl, Ca(OH)2, and a temporary filling material (eg, IRM Intermediate Restorative Material [Dentsply Sirona]). The use of these materials is the reason long-term prognosis following vital pulp therapy was poor.16 Also, we did not have a good understanding of the histological response of pulpal tissue to decay until Ricucci et al18 reported in the literature how the pretreatment pulpal diagnosis correlated to the clinical pulpal histology.
Vital pulp cryotherapy is a new technique that uses sterile ice, EDTA, BCs, and a permanent restoration (composite or amalgam).19,20 Vital pulp cryotherapy is done on teeth requiring a pulp cap or partial pulpectomy that can then be restored with an amalgam or composite restoration. Vital pulp cryotherapy is contraindicated when a tooth has a pretreatment periradicular diagnosis of asymptomatic apical periodontitis or chronic/acute apical abscesses or when the pretreatment pulpal diagnosis is necrotic.19 It is important to note that upon clinical access into a multi-rooted, “vital” tooth, if a clinician observes that the pulp is partially necrotic, it is also a contraindication for vital pulp cryotherapy treatment. Vital pulp cryotherapy is done under local anesthesia and using a rubber dam. If the pulp is exposed or indirectly exposed as a result of removing all the caries, as clinically demonstrated by the visual “blushing” of the pulp through a thin layer of dentin, vital pulp therapy is indicated. Shaved sterile-water ice (0° C) is then placed over a direct or indirect pulp exposure (Figure 3) for approximately 1 minute. No NaOCl should be applied to a direct pulp exposure when doing a vital pulp cryotherapy procedure. The reason for this is that NaOCl will kill the dental pulpal stem cells. EDTA solution should be used instead. EDTA has been shown to stimulate dental pulpal stem cells.21 After the melted sterile ice is suctioned away, 17% EDTA should be irrigated over the direct or indirect pulp exposure. It is not recommended to use an EDTA-soaked cotton pellet because the fibers from the cotton can remain after usage and be a source of inflammation of the pulp tissue. After EDTA irrigation, the direct or indirect exposed pulp is then covered with a BC material (Figure 4).
After completing a BC pulp cap or partial pulpectomy, a light-cured glass ionomer material or non-light-cured glass ionomer material should be placed directly over the BC pulp cap or partial pulpectomy.22 Next, a permanent restoration (composite or amalgam) is placed (Figure 5).
If the tooth cannot be permanently restored with a composite or amalgam, vital pulp cryotherapy is contraindicated, and the tooth should be treated with a full pulpectomy (regenerative endodontics).
REGENERATIVE ENDODONTICS
Regenerative endodontics is defined as biologically based procedures designed to physiologically replace damaged tooth structures, including dentin and root structures, as well as the pulp-dentin complex.23 This procedure is done when a full pulpectomy is indicated. It should be noted that in the endodontic literature, regenerative, revascularization, and revitalization endodontics are used synonymously and interchangeably.24 Reports in the literature have also demonstrated the use of regenerative endodontic procedures on not only immature permanent teeth with necrotic pulps but also on mature permanent teeth with necrotic pulps, teeth with persistent apical periodontitis after conventional endodontic treatment, traumatized teeth with external inflammatory resorption, teeth with horizontal fractures, and avulsed teeth.25-27 Regenerative endodontics has also been done on permanent, mature vital teeth (ie, pretreatment pulpal diagnoses of symptomatic irreversible pulpitis).28
In regard to regenerative endodontic canal preparation, it is important to address residual canal debris.14 Since it is understood that rotary NiTi files stay centered in a canal, the field of endodontics has reported in the literature the use of various irrigation techniques and devices (ultrasonic, negative pressure, syringe, and photo-induced photo-acoustic streaming [PIPS]) to assist in chemomechanical canal preparations.29 Recently, the literature has reported on the use of the multi-sonic GentleWave system (Sonendo) (Figure 6).29-31 These studies have found that canals were significantly cleaner with the use of the GentleWave system compared to the various irrigation devices mentioned above.29-31
There are some additional canal preparation guidelines that need to be followed when doing regenerative endodontics. First, the coronal two-thirds of the canal does not need to be overly enlarged. The reason for this is that the dentist does not have to be concerned with this portion of the canal being large enough to allow obturation instruments to gain access into the apical third of the canal. The coronal two-thirds of the canal’s conservative enlargement will also help to not weaken the tooth and thus prevent root fractures.32
Secondly, the final canal size of the apical foramen needs to be a minimum MAF size of 0.32 mm in order to allow the blood cells of the periapical tissue to migrate up into the canal space.16
Thirdly, during the file canal preparation stage, the canal should be irrigated with 1.5% NaOCl (a lower concentration is used to reduce the killing of stem cells)16 and an EDTA gel (EndoGel [Jordco] or RC-Prep [Premier Dental]) should be placed on each file prior to canal placement.33
Lastly, in teeth that have a pretreatment pulpal diagnosis of necrosis, it is recommended to treat these teeth in 2 visits. In these cases, after the canal preparation is completed, it is irrigated with 17% EDTA, dried, and temporized with the placement of Ca(OH)2 in the canal to serve as an antimicrobial medication between treatments.16 The canal access is temporized with a sterile cotton pellet or sponge and a temporary material (eg, Cavit Temporary Filling Material [3M], IRM Intermediate Restorative Material, or a glass-ionomer). The patient is then asked to return in 1 to 4 weeks for the final treatment appointment.16
On the second appointment for a necrotic tooth, after the Ca(OH)2 medicament has been removed from the canal(s), or on the first appointment for a vital tooth, the canal(s) need to be flushed with 17% EDTA irrigation after canal preparation. Bleeding is then induced with a hand file 1 to 2 mm beyond the apical foramen. The bleeding should be stopped at a level in the canal (cemento-enamel junction) to allow for 3 to 4 mm of restorative material. The induction of periapical bleeding into the canal space is necessary in regenerative endodontic procedures. This induced bleeding brings scaffolding, stem cells, and blood-derived bioactive growth factors. These growth factors are in addition to the growth factors embedded in the dentinal matrix that are released when the 17% EDTA is irrigated into the canal.21,34 The triad of stem cells, scaffolding, and growth factors is what enables the tissue regeneration to occur.35
A resorbable collagen matrix is placed over the blood clot. A bioceramic material is then placed directly over the collagen matrix, and a glass ionomer is placed over the bioceramic material. A final composite or amalgam restoration is then placed over the glass ionomer, and a final radiograph is taken. The patient should then be put on 3-month recall for up to 3 years, as healing dictates (Figure 7).28 The primary goal of bioactive endodontic treatment is the elimination of symptoms, the secondary goal is radiographic healing, and the tertiary goal is a positive response to sensitivity testing (Cold/Electric Pulp Tester [EPT]).28 It is important to note that with this paradigm shift in conventional endodontic treatment, the radiographic look of a completed root canal procedure will appear significantly different when compared to that of current conventional endodontic treatment.36
IN SUMMARY
In reassessing our current endodontic treatment, the future direction of endodontics is bioactive endodontics. This treatment is achieved by doing either vital pulp cryotherapy or regenerative endodontics. Vital pulp cryotherapy is a new procedure that uses sterile ice to reduce pulpal inflammation, in conjunction with EDTA and a BC material. Regenerative endodontics provides a more biological approach to conventional endodontic treatment than the current clinical methodology. This treatment technique involves canal preparation, followed by the induction of bleeding, from the periapical region into the canal. This procedure eliminates the use of warm vertical and cold lateral compaction techniques and carrier-based root-filling materials for canal obturation. The tissues generated in the canals after a regenerative endodontic procedure are cementum-like, bone-like, periodontal ligament-like tissues with blood vessels and nerves. Again, it is important to restate that although these tissues are not true pulpal tissue, they are the host’s own vital tissue as opposed to the use of foreign obturation materials (gutta-percha and sealer).
Acknowledgments:
The authors would like to thank Drs. Yagnik Patel and Nisreen Jaweesh, second-year endodontic residents at the University of Illinois at Chicago College of Dentistry, and Dr. Gail Tischke, in private practice limited to endodontics in Naperville, Ill, for the clinical treatment and documentation of the vital pulp cryotherapy and regenerative endodontic procedures documented in this article.
References
- Christensen CM, Hall T, Dillon K, et al. Competing Against Luck: The Story of Innovation and Customer Service. New York, NY: HarperCollins; 2016.
- Leong DJ, Yap AU. Quality of life of patients with endodontically treated teeth: a systematic review. Aust Endod J. 2019 Aug 20. [Epub ahead of print]
- Walia HM, Brantley WA, Gerstein H. An initial investigation of the bending and torsional properties of Nitinol root canal files. J Endod. 1988;14:346-351.
- Neelakantan P, Herrera DR, Pecorari VG, et al. Endotoxin levels after chemomechanical preparation of root canals with sodium hypochlorite or chlorhexidine: a systematic review of clinical trials and meta-analysis. Int Endod J. 2019;52:19-27.
- Siqueira JF Jr, Lopes HP. Mechanisms of antimicrobial activity of calcium hydroxide: a critical review. Int Endod J. 1999;32:361-369.
- Bystrom A, Sundqvist G. The antibacterial action of sodium hypochlorite and EDTA in 60 cases of endodontic therapy. Int Endod J. 1985;18:35-40.
- Bahcall J, Barss J. Orascopic visualization technique for conventional and surgical endodontics. Int Endod J. 2003;36:441-447.
- Saunders WP, Saunders EM. Conventional endodontics and the operating microscope. Dent Clin North Am. 1997;41:415-428.
- Scarfe WC, Levin MD, Gane D, et al. Use of cone beam computed tomography in endodontics. Int J Dent. 2009;2009:634567.
- Chandler N. Electronic apex locators may be better at determining endodontic working length than radiographs and could reduce patient radiation exposure. J Evid Based Dent Pract. 2015;15:28-29.
- Bahcall JK, Barss JT. Understanding and evaluating the endodontic file. Gen Dent. 2000;48:690-692.
- Gundappa M, Bansal R, Khoriya S, et al. Root canal centering ability of rotary cutting nickel titanium instruments: a meta-analysis. J Conserv Dent. 2014;17:504-509.
- McRay B, Cox TC, Cohenca N, et al. A micro-computed tomography-based comparison of the canal transportation and centering ability of ProTaper Universal rotary and WaveOne reciprocating files. Quintessence Int. 2014;45:101-108.
- Lin LM, Skribner JE, Gaengler P. Factors associated with endodontic treatment failures. J Endod. 1992;18:625-627.
- Weine FS. Endodontic Therapy. 6th ed. St. Louis, MO: Mosby; 2004.
- Saoud TM, Ricucci D, Lin LM, et al. Regeneration and repair in endodontics—a special issue of the regenerative endodontics—a new era in clinical endodontics. Dent J (Basel). 2016;4(1). pii: E3.
- Frank AL, Abou-Rass M, Glick DH. Changing trends in endodontics. J Am Dent Assoc. 1978;96:202-210.
- Ricucci D, Loghin S, Siqueira JF Jr. Correlation between clinical and histologic pulp diagnoses. J Endod. 2014;40:1932-1939.
- Bahcall J, Johnson B, Xie Q, et al. Introduction to vital pulp cryotherapy. Endodontic Practice US. 2019;11:12-14.
- Bahcall J, Xie Q, Baker M, et al. New approaches in endodontics: cryotherapy for vital pulp treatment. Decisions in Dentistry. 2019;5:14-18.
- Galler KM, Buchalla W, Hiller KA, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41:363-368.
- Wang Z. Bioceramic materials in endodontics. Endod Topics. 2015;32:3-30.
- Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endod. 2007;33:377-390.
- Kim SG, Malek M, Sigurdsson A, et al. Regenerative endodontics: a comprehensive review. Int Endod J. 2018;51:1367-1388.
- Saoud TM, Martin G, Chen YH, et al. Treatment of mature permanent teeth with necrotic pulps and apical periodontitis using regenerative endodontic procedures: a case series. J Endod. 2016;42:57-65.
- Saoud TM, Huang GT, Gibbs JL, et al. Management of teeth with persistent apical periodontitis after root canal treatment using regenerative endodontic therapy. J Endod. 2015;41:1743-1748.
- Santiago CN, Pinto SS, Sassone LM, et al. Revascularization technique for the treatment of external inflammatory root resorption: a report of 3 cases. J Endod. 2015;41:1560-1564.
- Bahcall J, Xie Q, Baker M, et al. Bioactive endodontics for mature permanent teeth. Inside Dentistry. 2019;15:43-46.
- Sigurdsson A, Garland RW, Le KT, et al. 12-month healing rates after endodontic therapy using the novel GentleWave System: a prospective multicenter clinical study. J Endod. 2016;42:1040-1048.
- Sigurdsson A, Le KT, Woo SM, et al. Six-month healing success rates after endodontic treatment using the novel GentleWave System: the pure prospective multi-center clinical study. J Clin Exp Dent. 2016;8:e290-e298.
- Molina B, Glickman G, Vandrangi P, et al. Evaluation of root canal debridement of human molars using the GentleWave System. J Endod. 2015;41:1701-1705.
- Khoshbin E, Donyavi Z, Abbasi Atibeh E, et al. The effect of canal preparation with four different rotary systems on formation of dentinal cracks: an in vitro evaluation. Iran Endod J. 2018;13:163-168.
- Bahcall J, Johnson B. Clinical guide to treating endodontic emergencies. Inside Dentistry. 2016;12:46-52.
- Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014;40:521-525.
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920-926.
- Bahcall J, Xie Q, Baker M, et al. The changing look of clinical endodontics. Inside Dentistry. 2019;15:46-50.
Dr. Bahcall is a clinical professor in the department of endodontics at the University of Illinois at Chicago (UIC) College of Dentistry. He can be reached at jbahcall@uic.edu.
Dr. Xie is an assistant professor in the department of endodontics at the UIC College of Dentistry. He can be reached via email at xieqian@uic.edu.
Dr. Baker is a clinical associate professor in the department of endodontics at the UIC College of Dentistry. He can be reached via email at mbaker@uic.edu.
Dr. Weeks is a clinical assistant professor in the department of endodontics at the UIC College of Dentistry. He can be reached via email at sweeks@uic.edu.
Disclosure: The authors report no disclosures.
Related Articles
Continuous or Reciprocating Endodontic Rotary Files: Evidence-Based Clinical Considerations
Overcoming Endodontic Procedural Failures: Part 2
The Set Up: Endodontic Predictability