Dental professionals can learn lessons from biofilms. Biofilms are both resilient and adaptive. They often show remarkable organization and can communicate, coordinate, and cooperate with each other. In times of change and challenge, this cooperation enhances their individual fitness and increases the efficiency of the biofilm. Dentistry can create similar synergies with the rest of healthcare through the use of interoperable electronic health records (EHRs). Healthcare Silos
Dentistry is in a period of transformation. An aging, more diverse population; the Affordable Care Act; and consumers relying more on technology and seeking a greater value from their spending have all placed dentistry in a state of flux.1 Each part of our complex healthcare community makes a difference. Cross-discipline and interprofessional collaboration can create successful partnerships that result in better patient care. Dentists can and should participate in interdisciplinary medicine, but our avoidance of EHRs maintains dental silos that continue to isolate professionals.
Interoperable EHRs can break down these silos. Many individual practitioners and practices are in transition. In the near future, Minnesota practitioners will no longer have the choice. Minnesota dentists, oral surgeons, and orthodontists are required to adopt a certified, interoperable EHR system, like their medical counterparts, by 2015.2 The greatest potential benefit of interoperable EHR is to break down silos to share collective knowledge. Like biofilms, working together requires communication and cooperation.
 |
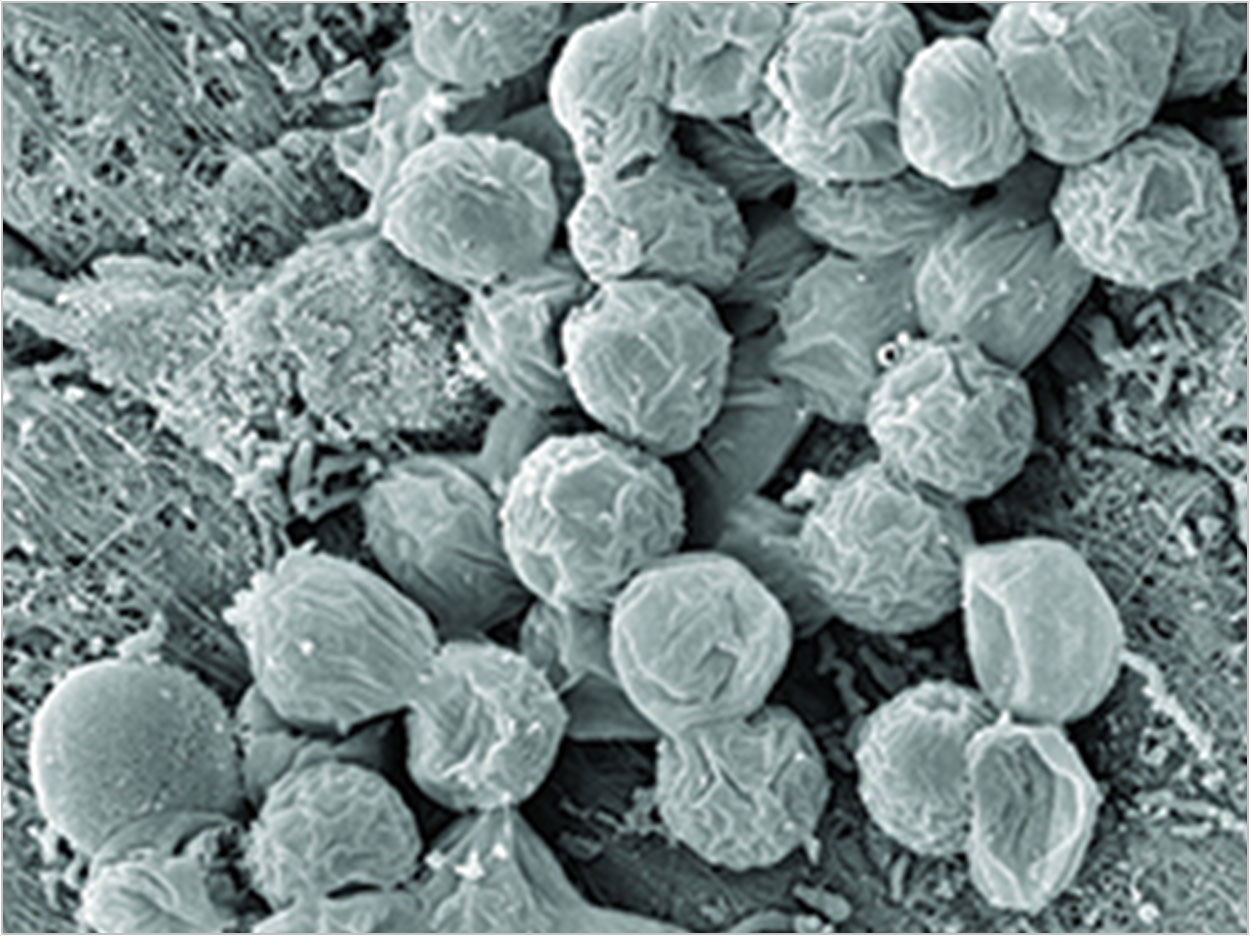
| Figure 1. Mixed specifics biofilm from a periodontal pocket. |
 |
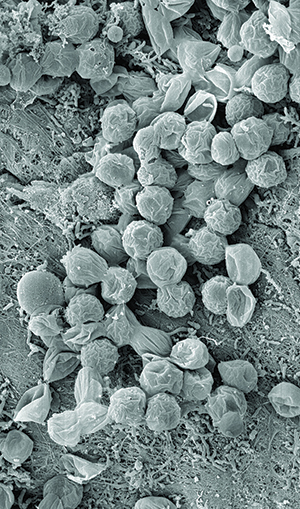
| Figure 2. The unique ecological niche of periodontal disease. |
 |
| Figure 3. Mixed specifics biofilm from a periodontal pocket. |
Periodontal Wound
The words biofilm and plaque are used interchangeably. Plaque is a form of biofilm, but biofilms are not limited to plaque formation; biofilms are more varied in makeup and location than plaque generally is. Many of us completed our education at a time when bacteria were studied in a free-floating planktonic state. This led to the concept that certain pathologic pathogens were the primary reason for the oral breakdown. Microbes living in a nonadherent and free-floating planktonic state cause acute diseases. Chronic disease, however, is often caused by a community of biofilms.3 An oral biofilm is an accumulation of a mixed population of bacteria (typically an oral biofilm has more than 500 bacterial species)4 that can also accommodate fungi, or protozoa, all of which is covered and protected by large amounts of slime or matrix material produced by the community. Through the work of Bill Costerton, referred to as the “father of biofilms,” we know that the complex makeup of the biofilm community and its ability for self-preservation is amazing.5
Periodontal disease starts with a biofilm-based infection that creates a wound that induces the inflammatory cascade responsible for the disease. The wounds should be taken seriously themselves. Mealey and Rose6 discuss the periodontium as a unique ecological niche in the human body. Prior to the eruption of teeth, the tissue is intact, yet inhabited by bacterial communities that don’t challenge the individual’s oral health, similar to the bacteria that thrive harmlessly on the skin. When the teeth erupt, this surface can have as many as 32 objects violating this formerly intact mucosa, creating the potential for biofilm and promoters of inflammation to reach the bloodstream (Figure 1).
When patients present with inflamed periodontal wounds, they are often treated without considering the biology of wounds and wound healing.7 Medicine has many guidelines on wound care from the US Department of Health and Human Services for Local Wound Care, the Association for the Advancement of Wound Care, as well as hundreds wound care textbooks for a variety of medical disciplines.
Antibiotic Resistance
Care of wounds in both dentistry and medicine has centered on the use of antibiotics. In the past 60 years, antibiotics have been crucial in the fight against infectious disease. They became thought of as miracle drugs and magic bullets. This magic bullet thinking has led to overprescribing and misuse, creating a resurgence of infectious diseases and the appearance of new ones. Whenever antibiotics wage war on micro-organisms, a few are able to survive the drug. Because microbes are always mutating, some eventually will protect against the drug. When antibiotics are used unnecessarily, they add to the selective pressure we are putting on microbes to evolve resistance. Then, when we really need antibiotics, they are less effective.8-10 Still, both dentistry and medicine often continue to rely on these strategies even when we recognize that the bacterial game has changed from single planktonic players to well-organized and defensive teams of biofilm. The main problem is that we need new options. Being more selective in our use of antibiotics is a first step. It has led to better outcomes in diabetic wound care.
New Views from Diabetic Wound Care
Diabetic foot ulcers are the most common, disabling, and costly complications of diabetes. Like periodontal disease, they are chronic infections. Diabetic wounds are very difficult to heal and are often considered the beginning of the end for many patients. In 2006, the Centers for Disease Control’s estimates showed 65,700 lower-limb amputations were performed in people with diabetes.11 Of those amputees, 70% will die within 5 years. Patients interviewed rated their quality of life as worse than cancer patients.
The majority of medical wound management protocols do not account for the discoveries of biofilm. Treatments are mostly based on an understanding of planktonic bacteria that can be cultured. Robert Koch, more than 100 years ago, was only able to study single bacterial organisms at a time because that is what grows on an agar plate. Koch’s postulates became the theoretical basis for wound care.12 Planktonic bacteria usually can be killed by an antibiotic. But bacteria organizing and protecting themselves in biofilms are more difficult to treat. Using antibiotics against a biofilm infection may not be effective and can add to greater resistance.
A pioneering physician, Dr. Randy Wolcott of the Southwest Regional Wound Care Center in Lubbock, Tex, believed that there had to be a different way than continuing to take patients down a path that led to death. His biofilm education, like many professionals, came through Dr. Costerton and the Center for Biofilm Engineering (visit the Web site biofilm.montana.edu). Dr. Wolcott took samples from various places in individual patients’ diabetic wounds. Rather than performing standard culture testing, he chose to send his samples to the Center. It was determined that the samples were largely multimicrobial biofilms.13 A full body of research is developing around these wound care findings. It became clear the both the testing through routine cultures and therapies needed to change to improve outcomes.
A key to Dr. Wolcott’s wound care silo story is the decision not to accept current protocols as the only choice. He recognized cultures are inadequate for identifying bacteria in biofilms that are multimicrobial. Polymerase chain reaction (PCR) testing, developed in 1983, is now a common and an often indispensable technique used in medical and biological research labs for a variety of applications.14-17
Testing is still not standard practice in dentistry. It is the experience of this author for periodontal disease to remain undiagnosed until the loss alveolar bone is evidenced. Dr. Wolcott sought a different path for his patients before the loss of a body part.
No One-Step Approach
There is no one-step solution for treatment of managing biofilm wounds. A combination approach is needed that aims to:
- Reduce the biofilm burden.
- Prevent reconstitution of the biofilm.
Dr. Wolcott’s novel approach starts with wound debridement with a curette and other chemical agents then the use of hyperbaric chambers with a super-oxygenated environment. Based on the result of a microbial PCR-DNA analysis, a customized antibiotic medicament is applied. Multiple therapeutic care appointments are often needed to promote healing. Wolcott’s lesson is that systemic antibiotics are not helpful for this kind of chronic wound infection.
Dentistry has managed biofilm with a well-known daily regimen of debridement (brushing) at the same time applying antibiofilm substance-toothpaste. These antibiofilm agents block reattachment or are biocidal, killing the community members of the biofilms. For more recalcitrant biofilms, harsher biocides are applied through oral rinses. Aggressive debridement can be carried out through professional ultrasonic and hand instrumentation. Is it enough? Do dental professionals have to be satisfied? What can dentistry learn from Dr. Wolcott?
Different Approaches for Different Outcomes
The most important takeaway from Dr. Wolcott’s story is the importance of addressing the biofilm-induced wound chemically and with oxygen. Perio Protect offers a novel option for periodontal wound treatment that parallels Dr. Wolcott’s lead and that can potentially lead to improved outcomes.
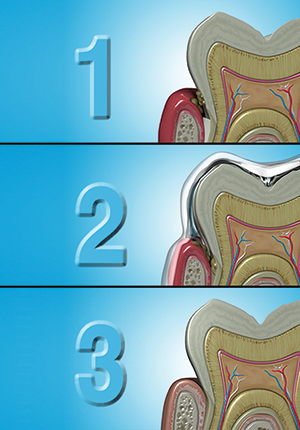
Perio Protect (perioprotect.com) is a comprehensive method that is customized for individual patients to help manage biofilms. It is a combination of treatments, including a noninvasive chemical debriding therapy used in conjunction with traditional mechanical debriding procedures. The Perio Tray (Figure 2) is a prescription medical device that places medication in the sulcus or periodontal pocket. The most common medications prescribed with Perio Trays are oral debriding agents, antibiotics, and fluorides to address biofilm infections, inflammation, root caries, or recession-induced sensitivity. The trays have customized seals and extensions fabricated along the interior periphery of the Perio Tray that prevent medication from leaking into the oral cavity and provide positive pressure to direct medication deep into the sulcus and periodontal pocket. Research shows, in pockets as deep as 8.0 mm, significant drops in bleeding on probing and pocket depths for patients using Perio Tray delivery.18-19 Because Perio Trays are classified as prescription medical devices, they require a doctor’s script and must be fabricated in a dental laboratory registered with the FDA and trained by Perio Protect.
Ozone therapy has been used in many countries throughout the world for more than 100 years. Clinical research is currently being conducted and monitored from multiple practice-based research network clinical centers throughout the United States. As a result of the research findings, foundational protocols have been developed to address common oral infections such as periodontal disease, endodontic infections, dental decay, osteomyelitis, bisphosphonate induced osteonecrosis, stomatitis, and herpetic lesions.20 When ozone is introduced into a living system, and it creates a transient oxidative burst, the micro-organisms that have no natural defensive against this reaction are overstressed and die. In cases where periodontal therapy involves calculus removal, the sulci and pockets are initially irrigated with ozonated water in a syringe/cannula configuration. After treatment, each pocket is then treated with ozone gas from a generator located in the treatment room. This oxygenation treatment further reduces the pathogenic organisms as well as stimulates tissue healing.
Closing Comments
Biofilms are complex communities of different species. Under natural conditions, they tend to attach and form a community (Figure 3). By sharing genetic material, they differentiate and change their gene expressions. Through quorum sensing, they signal other species to join them. Together, they excrete a protective layer, making them difficult to break up. Biofilms share “skills and abilities” for the survival of the group.3 Biofilms are dynamic and ever-changing; the whole is greater the sum of its parts. All species in a biofilm are important because they can act synergistically. Working together requires communication and cooperation.
Healthcare is a complex community with different specialties. Each part makes a difference. Cross-discipline and interprofessional collaboration can create successful partnerships that result in better patient care. Healthcare can begin share skills, abilities, and knowledge through EHR systems to learn and grow then to act synergistically with the sum becoming more than its individual parts. It’s time to drop biased silo strategies ingrained throughout the years. The game has changed and so must healthcare; the opportunity is here.
References
1. Diringer J, Phipps K, Carsel B. Critical Trends Affecting the Future of Dentistry: Assessing the Shifting Landscape. May 2013. ada.org/sections/professionalresources/pdfs/Escan2013_Diringer_Full.pdf. Accessed March 13, 2014.
2. Minnesota Department of Health. Guidance for understanding the Minnesota 2015 Interoperable EHR Mandate. health.state.mn.us/e-health/hitimp/2015mandateguidance.pdf. Accessed March 13, 2014.
3. Proal A. Understanding biofilms. May 26, 2008. bacteriality.com/2008/05/26/biofilm. Accessed March 13, 2014.
4. Marsh PD. Dental plaque as a biofilm and a microbial community—implications for health and disease. BMC Oral Health. 2006;6(suppl 1):S14.
5. Costerton JW. New ammunition. Dimensions of Dental Hygiene. 2007;5:14-16.
6. Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Compend Contin Educ Dent. 2008;29:402-413.
7. Gurenlian JR. The role of dental plaque biofilm in oral health. cdeworld.com/courses/20009-The_Role_of_Dental_Plaque_Biofilm_in_Oral_Health. Accessed March 13, 2014.
8. Keller D. Dental oral wound clinic. aaoshconnect.org/issue/july-2013/article/dental-oral-wound-clinic. Accessed March 13, 2014.
9. Todar K. Bacterial resistance to antibiotics. In: Today’s Online Textbook to Bacteriology. textbookofbacteriology.net/resantimicrobial.html. Accessed March 13, 2014.
10. Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010;1:134.
11. Centers for Disease Control and Prevention. 2011 National Diabetes Fact Sheet. cdc.gov/diabetes/pubs/estimates11.htm. Accessed March 13, 2014.
12. Ehrlich GD, Arciola CR. From Koch’s postulates to biofilm theory. The lesson of Bill Costerton. Int J Artif Organs. 2012;35:695-699.
13. Proal A. Interview with Dr. Randall Wolcott, bacterial biofilm wound specialist. April 13, 2008. bacteriality.com/2008/04/13/wolcott. Accessed March 13, 2014.
14. Infection update: Methods for microbial identification in chronic wounds. Wounds International. 2012;3:1-6.
15. Rhoads DD, Wolcott RD, Sun Y, et al. Comparison of culture and molecular identification of bacteria in chronic wounds. Int J Mol Sci. 2012;13:2535-2550.
16. Wolcott R, Dowd S. The role of biofilms: are we hitting the right target? Plast Reconstr Surg. 2011;127(suppl 1):28S-35S.
17. Phillips PL, Wolcott RD, Fletcher J, et al. Biofilms made easy. Wounds International. 2010;1:1-6.
18. Putt MS, Proskin HM. Custom tray application of peroxide gel as an adjunct to scaling and root planing in the treatment of periodontitis: results of a randomized, controlled trial after six months. J Clin Dent. 2013;24(3):100-107.
19. Dunlap T, Keller DC, Marshall MV, et al. Subgingival delivery of oral debriding agents: a proof of concept. J Clin Dent. 2011;22:149-158.
20. Rothchild J, Harris B, Mollica P. Current concepts of oxygen ozone therapy for dentistry in the United States. International Journal of Ozone Therapy. 2010;9:105-108.
Ms. DiGangi continues to take an active, future-oriented leadership role in a variety of professional organizations. She is a certified Health Information Technology trainer and a member of the American Health Information Management Association, taking an active role in shaping the changes in our electronic world. She is an ADA Evidence-Based Champion and holds a publishing license with the ADA for current dental terminology. She is the author of the DentalCodeology series of minibooks. She can be reached via e-mail at info@pdigangi.com.
Disclosure: Ms. DiGangi recevied a research honorarium from PerioProtect for this article. She also has consulting positions with Air Techniques, Xlear, American Eagle, GC America, Solution Reach, VOCO America, Phocal/Colldent, Lexicomp, and Young Dental.












