The trigeminal nerve and its peripheral branches are susceptible to injury in the practice of dentistry. Neurosensory deficits can be debilitating to some patients due to their effects on speech, taste, mastication, and activities of daily living. Imagine the effect of food and liquid incompetence during a meal with business colleagues or diminished taste for a wine connoisseur.
The good news is that the vast majority of these peripheral trigeminal nerve injuries undergo spontaneous regeneration. However, some injuries may be permanent with varying degrees of sensory impairment ranging from mild numbness (hypoesthesia) to complete anesthesia. Some patients may even develop burning pain called dysesthesia in addition to their sensory deficits.
The goal of trigeminal nerve microsurgery is to restore nerve continuity by removing any obstacles such as scarring or foreign debris and creating nerve continuity. This paper will review mechanisms of trigeminal nerve injury in dentistry, clinical neurosensory testing, indications for surgery, and referral to a microsurgeon as well as surgical procedures that include adjunct materials to enhance regeneration and to improve activities of daily living for our patients.
Mechanisms of Trigeminal Nerve Injuries in Dentistry
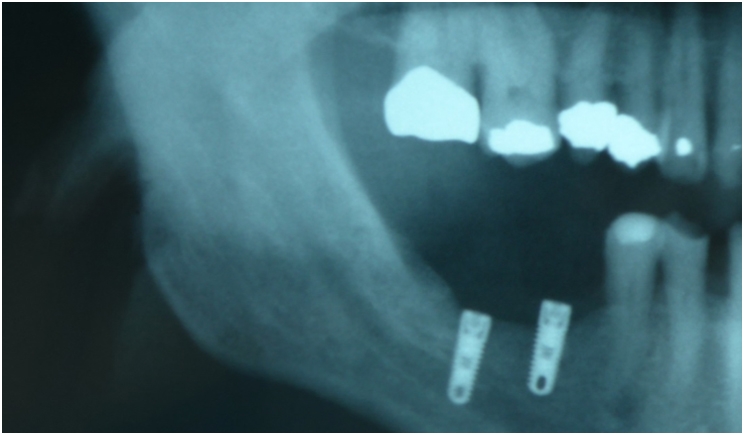
The most common procedures associated with trigeminal nerve injury in the practice of dentistry include the removal of impacted third molars and placement of endosseous dental implants (Figure 1). Additional causes can include endodontic procedures and even administration of local anesthesia. Other less common surgical procedures include orthognathic surgery with osteotomies, facial trauma, and management of maxillofacial pathology.
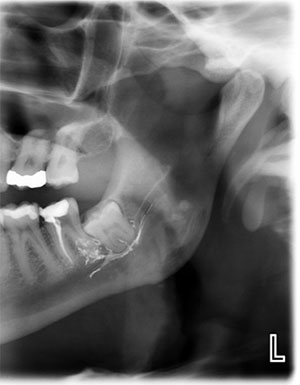
In our practice, the three most commonly observed etiologies for trigeminal nerve injuries include dental extractions, placement of implants, and endodontic procedures where endodontic materials are extruded into the inferior alveolar nerve canal (Figure 2).
 |
|
Figure 2. Endodontic materials extruded into the IAN such as this paste represent one of the three most commonly observed etiologies for trigeminal nerve injuries. |
 |
 |
|
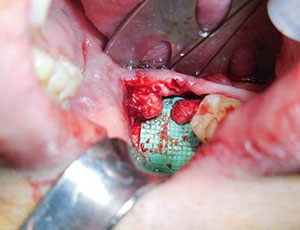
Figure 3. Preparation of the nerve for neurorrhaphy begins with the preparation of the proximal and distal nerve segments back to healthy nerve tissue noted by axoplasmic bulging, punctate bleeding, and lack of fibrosis. |
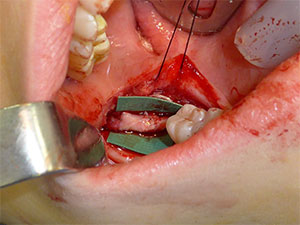
Figure 4. Allogenic nerve graft can be used to reconstruct defects when there is an inability to effect tension-free repair. |
Temporary or permanent sensory nerve disturbance associated with removal of impacted third molars including surgical exodontia is a result of lingual and/or inferior alveolar nerve injuries. In some cases, socket preservation techniques using allogeneic bone grafts result in bone being displaced into the nerve canal, which can cause nerve injuries as well.
Dentists must discuss the potential for these types of injuries during the informed consent process and document the discussion accordingly. Once identified, these injuries need to be followed on a regular basis with serial nerve testing to document any sensory changes. Neurosensory testing and indications for trigeminal nerve microsurgery will be discussed later in this paper.
Injury to the inferior alveolar nerve (IAN) from impacted third molar removal has been negatively correlated with several factors including older patient age, depth of impaction, development of root structure, angulation of impacted tooth, and location of root apex relative to the IAN. Operator experience also plays a role in the incidence of nerve injuries. Overzealous use of rotary instrumentation for bone removal and sectioning of teeth may lead to direct nerve injuries.
It is not uncommon for some clinicians to consider the use of intentional partial odontectomy (where the crown is removed and roots are retained) in those cases where IAN injury risk is high. There are limitations to this procedure in that tooth structure should not be retained in presence of infection or pathology. In addition, patients may develop secondary infection or migration of root fragments necessitating secondary surgery, which must be discussed with patient preoperatively.
That is why I reserve the intentional partial odontectomy procedure for only very select patients and often opt to remove complex third molars after advanced imaging in the operating room setting where concomitant microsurgery can be performed intraoperatively if indicated.
Lingual nerve injuries associated with third molar surgery can occur resulting in tongue biting, changes in speech such as lisping, and diminished taste perception. Patients may also describe alteration in taste with increased bitterness or metallic taste due to the predominance of the glossopharyngeal nerve distribution involving the posterior third of the tongue with diminished intensity of salt, sour, and sweet tastes, which are mediated by the anterior two thirds of the tongue.
Remember that the chorda tympani nerve, which mediates taste in the anterior two thirds of the tongue, runs along with the lingual nerve and can be injured concomitantly. The lingual nerve is not generally visualized during third molar surgery and may have a varying anatomic location. Anatomic studies have demonstrated the variation in location of the nerve with up to 10% of the patient’s nerve located above the lingual bone crest and in direct contact with the lingual plate in up to 25% of patients. This puts the lingual nerve at risk depending on if the incision is placed too far lingually or if the lingual plate is fractured or breached by surgical drill or hand instrumentation.
I have observed cases where the impacted third molar is lingually oriented, eliminating some of the lingual plate and placing the nerve in a precarious location during extraction, even if the nerve is located within normal anatomical variants. It must be stressed to be cognizant of incision design with buccal or lateral orientation and to not violate the lingual plate of bone with rotary instrumentation. The use of lingual flap retraction during third molar surgery may be associated with an increased incidence of temporary nerve damage due to traction injury.
Local anesthesia injections may cause trigeminal nerve injuries in some cases. Fortunately, most of these injuries resolve spontaneously, but some can be permanent. It has been estimated that approximately one in 100,000 injections results in a neurosensory deficit. This translates into one or two local anesthetic injection injuries during the career of an active dental practitioner. The exact etiology of this injury is unclear with several postulated mechanisms.
Direct trauma from a needle penetration of the nerve will illicit a painful response from the patient and cause the dentist to withdraw and re-direct the needle. It would be excruciatingly painful and unlikely to inject local anesthesia within the nerve sheath on an awake patient. In addition, the surface area of a needle perforation is very small relative to the diameter of the nerve and often disproportionate to the level of sensory deficit clinically observed.
A more plausible cause is the development of a barb on the needle due to contact with bone during repeated injections. If the needle then penetrates the nerve, it theorectically could cause an intraneural hematoma, which can lead to ischemia from pressure, fibrosis, and eventually permanent sensory deficit. An explanation for the varying degrees of sensory deficit that can result from injection injuries may be related to a decreased number of fascicles within the nerve at the location of injection by the lingula relative to the number of fascicles at the region of the third molar.
In addition to mechanical injuries, all local anesthetics are neurotoxic and have the potential to cause nerve injuries. The concentration of local anesthesia, presence of a vasoconstrictor, and penetrability of the local can all potentially affect the risk of injury. Some clinicians will opt to avoid use of more concentrated local anesthetics for inferior alveolar nerve blocks and reserve them for areas where infiltration can be performed.
Regardless of the cause for local anesthetic injection nerve injuries, there is no reliable way to prevent this injury from occurring. Good technique involves slowly injecting patients and reliance upon aspiration before injection. Any injury resulting from local anesthesia injections must be documented and monitored with serial examinations.
Patients can be reassured that most of these injures will resolve spontaneously. In the rare circumstance where patients develop neuropathic pain and dysesthesia that become centralized, pharmacologic management and referral to a pain management specialist is indicated. Trigeminal nerve microsurgery has not proven to be successful in these cases and is generally not indicated for patients with local anesthesia injuries.
Dental implant placement can result in injury to the IAN from the region of the mental nerve where an anterior loop is present to anywhere along the path of the nerve. Implant injuries can occur from a variety of mechanisms including direct mechanical compression from the implant, fracture of cortical bone with displacement into the canal, or drilling of the osteotomy and bleeding within the canal leading to essentially a compartment syndrome within the canal by compressing the nerve.
Thorough preoperative planning with appropriate imaging, use of computer generated guides, and probing of osteotomy sites to ensure intact bone structure can minimize these injuries. Postoperative radiographs should be standard practice after implant placement. If the implant appears to impinge on the nerve canal, it should either be removed and replaced with a shorter implant or backed out appropriately. Nerve lateralization techniques to allow placement of longer implants have been utilized in the past. However, the procedure itself was responsible for some permanent sensory deficits.
Endodontic treatment can result in trigeminal nerve injuries, which can be devastating. Over-instrumentation of the canal system can provide a direct conduit for mechanical injuries by files or gutta-percha materials. Chemical injury can be caused by sterilizing solutions including sodium hypochlorite and cements that often contain eugenol, both of which are neurotoxic.
Direct trauma can also occur from endodontic surgery such as apicoectomies on posterior teeth where roots are in close proximity to the IAN. Endodontic material displacement into the IAN canal should always be viewed as an emergency.
In addition to sensory deficits, many patients can develop neuropathic pain, which has limited benefit from trigeminal nerve microsurgery. These patients should be referred to a surgeon experienced in microsurgery to remove any foreign materials as soon as possible before the onset of neuropathic pain.
Clinical Neurosensory Testing
Once a neurosensory deficit has been diagnosed, neurosensory testing is indicated to quantify the degree of sensory impairment, monitor any spontaneous sensory recovery, and determine if trigeminal nerve microsurgery is indicated. The affected area is mapped out on the patient’s face and recorded on a suitable diagram for reproducible recordings of serial examinations.
A light brush or sterile needle can be used to delineate the affected area. The contralateral or unaffected side is used as a control, and all measurements are recorded. For bilateral injuries, an unaffected adjacent facial region is used as the control. Adequate pressure is applied to elicit a response. Penetration of the skin provides no useful additional information. Three levels of testing are performed for patients with hypoesthesia or numbness.
Level A includes two-point discrimination and directional sense. Two-point discrimination can be performed using a caliber or boley gauge. Normal values are approximately 3 to 4 mm. Values greater than 20 mm are generally not recorded since contralateral side innervation will start to play a role at this distance. Directional sense is achieved using a light brush or thin nylon monofilament and assessing whether the patient can determine stroke and direction.
Level B testing is used to evaluate non-noxious stimuli such as vibration and static light touch. Vibration is easily measured using a tuning fork, and static light touch is measured with von Frey monofilaments. The nylon monofilament is applied perpendicular to the skin, and pressure is applied until the filament bends. The thicker diameter filament will require more force to bend. The filaments are calibrated based upon the pressure required to bend, and a quantitative value can be recorded.
Level C testing is used to measure noxious stimuli including pain and temperature. Pain is assessed using a sterile needle to gently touch skin to determine if a sharp or painful sensation is perceived. Hot and cold sensation can be evaluated using a cotton-tipped applicator with hot water and ethyl chloride spray. A pressure algesiometer may also be used to provide a standardized amount of pressure for each measurement. The different tests correspond to specific nerve fibers that mediate each of the sensations.
Current perception threshold is a technique more commonly used in research where affected areas are stimulated by an electrical stimulus. The frequency of the stimulation correlates to specific nerve fibers, and patients are able to quantitatively record their threshold response to increasing intensity of current. This technique enables the researcher to quantitatively assess nerve injuries, although this has been shown to correlate well to that of traditional clinical neurosensory testing.
The same testing procedures can be used for patients with neuropathic pain with the goal of characterizing the response to varying stimuli. Level A testing where gentle stroking of the skin elicits pain is called allodynia. Level B testing determines if the patient has hyperpathia, which is defined as abnormal painful response with delayed onset, increasing intensity with repeated stimuli, and persistent sensation after stimulation is removed. Level C testing will determine if hyperalgesia or increased sensitivity that is not proportional to the stimulation is present. For patients with pain, local anesthetic blocks can be utilized to determine whether the injury is peripheral by determining if they are effective in reducing or eliminating pain.
Indications for Trigeminal Nerve Microsurgery
A neurosensory disturbance that persists for more than three months is a generally accepted indication for exploratory trigeminal nerve microsurgery. However, this concept will likely change in the future with the availability of high-resolution MRI studies including magnetic resonance neurography, where nerve injuries can be directly visualized, eliminating the need to wait in cases of severe or complete injuries.
Indications for trigeminal nerve microsurgery include:
- Observed nerve transection
- No subjective sensory improvement for longer than 3 months
- Development of new onset pain in the affected region
- The presence of a foreign body
- Progressively worsening hypoesthesia or dysesthesia
- Hypoesthesia that is intolerable to the patient.
The presence of a foreign body such as in the case of endodontic filling materials within the nerve canal is an indication for immediate exploratory microsurgery prior to the onset of neuropathic pain.
Contraindications for trigeminal microsurgery would include:
- Evidence of improving sensory function
- Hypoesthesia that is acceptable to the patient
- A severely medically compromised patient
- Central neuropathic pain
- Excessive time since injury
Studies have demonstrated significant sensory improvement after trigeminal nerve microsurgery from six to nine months after the injury. However, the prognosis will generally be diminished over time.
Trigeminal Nerve Microsurgery
The basic surgical tenets involved in peripheral trigeminal nerve microsurgery include decompression and exposure of the nerve, hemostasis, removal of any foreign material in or around the nerve, resection back to healthy nerve tissue, and tension-free neurorrhaphy.
The IAN may be approached either intraorally or transcervically via a submandibular incision. The transoral approach is most commonly utilized with exposure of the nerve by decorticating the lateral cortex or alternatively via a sagittal split mandibular osteotomy. The later approach provides good access. However, occlusion must be re-established upon completion of microsurgery, and application of rigid internal fixation for bone stability and healing are required.
The lingual nerve is approached by either a paralingual or lingual gingival sulcular incision in a subperiosteal plane. The paralingual incision is completed with blunt and sharp dissection along the floor of the mouth in the anticipated vicinity of the nerve to allow exposure. Advantages of this approach include a smaller incision with direct visualization of the nerve. However, a nerve that has sustained a complete injury may result in retraction of the proximal and distal segments upon exposure.
The lingual gingival sulcus incision requires a lateral release along the external oblique ridge for complete flap mobilization and elevation of the flap in a subperiosteal plane. Once the flap is elevated, the nerve may be visualized through the overlying periosteum and bluntly dissected from within the flap. This technique requires a larger incision than the paralingual mucosal incision design. However, the proximal and distal nerve will not retract during surgical dissection, and it is my preferred method.
In all cases of peripheral trigeminal nerve microsurgery, external neurolysis should be performed to expose and free it of any scar tissue, foreign bodies, or restrictions from the tissue bed. This will expose the nerve, allowing for better assessment of the injury for definitive surgical planning. The separation of scar tissue and freeing of an intact nerve may be the only necessary procedure to allow for recovery of sensation. External neurolysis is usually performed under some magnification to assess the nerve and to isolate any pathology such as neuroma.
Internal neurolysis is a less common procedure than can be used when there is evidence of nerve fibrosis in addition to restriction or compression. This technique is not practiced by all surgeons since there is potential for some iatrogenic injury to occur as a result of the procedure itself. A longitudinal incision is created through the epineurium to expose the internal fascicular structures and assess the integrity of the nerve.
Placement of a longitudinal incision along the nerve is called an epifascicular epineurotomy, whereas complete excision of the epineurium is called an epifascicular epineurectomy. With the release of the epineurium, the nerve may be allowed to expand, indicating a successful internal decompression. When fibrosis is observed and no viable nerve tissue is present, the affected segment should be excised and the nerve prepared for neurorrhaphy.
Preparation of the nerve for neurorrhaphy begins with the preparation of the proximal and distal nerve segments back to healthy nerve tissue noted by axoplasmic bulging, punctate bleeding, and lack of fibrosis (Figure 3). Abnormal tissue is excised in small increments until normal nerve tissue is observed under magnification. The proximal and distal nerve ends are then aligned and assessed as to whether tension-free closure is possible.
Mobilization of the IAN may be enhanced by distal and proximal nerve dissection and sacrifice of the incisive branch to allow lateralization and mobilization after decortication of the IAN from the site of injury to the mental foramina. Lingual nerve mobilization can be maximized by further proximal and distal dissection. Neurorrhaphy for peripheral trigeminal nerve injuries may be performed at the level of the epineurium or perineurium, though most commonly it is completed at the level of the epineurium.
Clinically, there is no difference in outcomes for sensory nerve repair by the epineural versus the perineural technique, although there is potential for iatrogenic injury when attempting repair at the level of the fascicles. A suture diameter smaller than 7-0 of a non-reactive material such as nylon is selected to minimize proliferation of scar tissue and inflammatory response.
The most critical issue remains tension across the neurorrhaphy site, which must be minimized to achieve optimal outcomes since tension can lead to restriction of blood flow and ischemia of the nerve. If a tension-free repair cannot be achieved via direct neurorrhaphy, the conduit assisted repair technique can be utilized. This technique allows for the placement of tension reliving sutures at the edge of the conduit.
Conduits also serve several other important functions in nerve repair. These include protection of the neurorrhaphy sites, prevention of fascicle misalignment, and prevention of scarring down or binding of the nerve during healing. Many conduit materials have been described and utilized historically, but most surgeons today prefer resorbable collagen or porcine small intestinal submucosa conduits, which remodel to form a new tissue layer similar to epineurium. Regardless of which material is utilized, these conduits aid in nerve regeneration by allowing capillary ingrowth and diffusion of growth factors to the site of injury.
If the gap between the nerve ends is too large for either a direct neurorrhaphy or a conduit assisted repair, an interpositional nerve graft, which can be either autogenous or allogeneic, can be used (Figure 4). Availability of allogeneic nerve grafts has dramatically changed options for patients since they eliminate the concomitant morbidity associated with the harvest of autogenous grafts. Use of grafts without tension can actually enhance outcomes compared to primary repairs performed under tension. I prefer allogeneic nerve grafts, which are readily available and acceptable to patients compared to the harvest of autogenous grafts.
Sensory Re-education
Sensory re-education may be helpful in helping patients expedite the onset of their sensory recovery. Sensory re-education exercises performed with daily massage and stimulation of the affected area in conjunction with administration of vitamin B12 result in achieving functional sensory recovery (FSR) sooner than those patients not employing these modalities. This is not to say that the final outcome is different, only that FSR can be obtained sooner.
The goal of sensory re-education is to stimulate the peripheral receptors so the central nervous system can then begin processing this sensory input. I recommend sensory re-education training and vitamin B12 to all patients postoperatively.
Outcomes of Trigeminal Nerve Microsurgery
Most outcome data for the microsurgical repair of peripheral trigeminal nerve injuries is based upon case reports and series. Historically, there was no standardization in quantifying outcomes from nerve injuries until implementation of the Medical Research Council Scale (MRCS). This tool standardizes and quantifies clinical sensory outcomes, which is useful for comparisons between studies.
The factors associated with successful sensory outcomes from microsurgery include time from injury to surgery, lack of preoperative neuropathic pain, absence of foreign bodies around the nerve, tension-free primary repair, and use of conduit-assisted repair or nerve grafts when tension-free repair is not possible.
Patients need to be counseled as to realistic outcome expectations with regards to time frame to recovery and final sensory levels achievable based upon the preoperative variables. Trigeminal nerve microsurgery has been shown to be an effective modality for restoring sensation after peripheral trigeminal nerve injuries.
Future Directions and Conclusions
Trigeminal nerve microsurgery has been shown to be an effective surgical treatment in the management of peripheral trigeminal nerve injuries. In addition to the availability of conduits and allogeneic nerve grafts, other adjuncts are becoming available to microsurgeons such as platelet rich plasma, which is used to enhance wound healing through release of growth factors and other bioactive substances that initiate wound healing. Adipose derived stem cells are another adjunct that may promote nerve regeneration. These types of emerging materials and techniques will continue to evolve and will likely contribute to enhanced sensory recovery in the future.
Bibliography
Behnia H, Kheradvar A, Shabrohbi M. An Anatomic Study of the Lingual Nerve in the Third Molar Region. J Oral Maxillofac Surg. 58:649-651; 2000.
Dessouky R, Xi Y, Zuniga J, Chhabra A. Role of MR Neurography for the Diagnosis of Peripheral Trigeminal Nerve Injuries in Patients with Prior Molar Tooth Extraction. Am J Neuroradiolo. 39:162-169: 2018.
Devine M, Modgill O, Renton T. Mandibular Division Trigeminal Nerve Injuries Following Primary Endodontic Treatment. A case series. Australian Endodontic J. 43:56-65; 2017.
Dodson TB and Kaban LB. Recommendations for Management of Trigeminal Nerve Defects Based on a Critical Appraisal of the Literature. J Oral Maxillofac Surg. 55:1380-1386; 1997.
Lampert RC, Nesbitt TR, Chuang SK, Ziccardi VB. Management of Endodontic Injuries to the Inferior Alveolar Nerve. Quintessence Int. 47:581-587; 2016.
Meyer RA and Bagheri SC. Clinical Evaluation of Peripheral Trigeminal Nerve Injuries. Atlas Oral Maxillofac Surg Clin N Am. 19:15-33; 2011.
Miloro M, Halkias LE, Slone HW, et al. Assessment of the Lingual Nerve in the Third Molar Region Using Magnetic Resonance Imaging. J Oral Maxillofac Surg. 55:134-137; 1997.
Nizam SA and Ziccardi VB. Trigeminal Nerve Injuries: Avoidance and Management of Iatrogenic Injury. Oral Maxillofac Surg Cl N Am. 27:411-424; 2015.
Pogrel MA. The Results of Microneurosurgery of the Inferior Alveolar and Lingual Nerve. J Oral Maxillofac Surg. 60:485-489; 2002.
Pogrel MA, Jergensen R, Burgon E, Hulme D. Long-Term Outcome of Trigeminal Nerve Injuries Related to Dental Treatment. J Oral Maxillofac Surg. 69:2284-2288; 2011.
Pogrel MA and Thamby S. Permanent Nerve Involvement Resulting from Inferior Alveolar Nerve Blocks. JADA. 131:901-907; 2000.
Pogrel MA, Bryan J, Regezi J. Nerve Damage Associated with Inferior Alveolar Nerve Blocks. JADA. 126:1150-1155; 1995.
Shanti RM, Khan J, Eliav E, Ziccardi VB. Is there a role for a Collagen Conduit and Anti-Inflammatory Agent in the Management of Partial Peripheral Nerve Injuries. J Oral Maxillofac Surg. 71:1119-1125, 2013.
Wilson MT, Chuang SK, Ziccardi, VB. Lingual Nerve Microsurgery Outcomes using Two Different Conduits: A retrospective cohort study. J Oral Maxillofac Surgery. 75:609-615; 2017.
Yampolsky A, Ziccardi VB, Chuang SK. Efficacy of Acellular Nerve Allografts in Trigeminal Nerve Reconstruction. J Oral Maxillofac Surgery. 75:2230-2234; 2017.
Yilmaz Z, Ucer C, Scher E, Suzuki J, Renton T. A Survey of the Opinion and Experience of UK Dentists: Part 2: Risk of Assessment Strategies and the Management of Iatrogenic Trigeminal Nerve Injuries Related to Dental Implant Surgery. Implant Dent. 26:256-262; 2017.
Ziccardi VB and Assael LA. Mechanisms of Trigeminal Nerve Injuries. Atlas Oral Maxillofac Surg Clin N Am. 9:1-11; 2001.
Ziccardi VB, Hullett J, Eliav E, Gomes J. Physical Neurosensory Testing versus Current Perception Threshold Assessment in Trigeminal Nerve Injuries Related to Dental Treatment: A Retrospective Study. Quintessence Int. 40:603-609; 2009.
Ziccardi VB. Microsurgical Techniques for Repair of the Inferior Alveolar and Lingual Nerves. Atlas Oral Maxillofac Surg Clin N Am. 19:79-90; 2011.
Dr. Ziccardi is a professor, chair, and residency director with the Rutgers University School of Dental Medicine Department of Oral and Maxillofacial Surgery. He can be reached at (973) 972-7462 or ziccarvb@sdm.rutgers.edu.
Disclosure: Dr. Ziccardi is a consultant with Axogen, Alachua, Florida.
Related Articles
The 90-Day Window: Trigeminal Nerve Injuries and the Importance of Prompt Referral
Rutgers Researcher to Explore Genetic Roots of Trigeminal Neuralgia
Treatment Addresses Trigeminal Neuralgia Without Side Effects











