INTRODUCTION
Directed dentin conservation (DDC) refers to the preservation of dentin structures and may play an important role in increasing the survival rate of endo-dontically treated teeth. Dynamic navigation uses data from a CBCT scan to provide real-time visual feedback during access cavity preparation. Head-up display (HUD) technology for the surgical operating microscope has been used in different fields of medicine since the 1990s and is reported to enhance the ergonomics during surgical procedures.
This case report describes the use of dynamic navigation and a surgical operating microscope HUD for contemporary access cavity preparation and optimal DDC during nonsurgical endodontic treatment. The importance of DDC and the potential advantages and limitations of dynamic navigation and HUD technology for the surgical operating microscope are also described.
DDC refers to the preservation of selective dentin structures. Clark and Khademi1 suggested that the maintenance of selective dentin structure of high value promotes optimal strength in endodontically treated teeth (ETT). Valuable dentin regions include the pericervical dentin (PCD), “soffits,” and “trusses.” The PCD includes dentin located 4 mm above and below the crest of bone.
It has been proposed that the PCD plays a crucial role in transferring occlusal forces along the root and that maintaining intact PCD is arguably the single most important factor in achieving long-term retention of ETT.2 A “soffit” is a lip of undercut dentin tissue left after access cavity preparation at the level of the pulp chamber roof.
A “truss” is a band of pulp chamber roof dentin that braces the buccal and lingual structures of the tooth and promotes resistance to tensile and compressive forces. Both soffits and trusses may contribute to the overall strength of ETT.3
The use of a preoperative CBCT scan to determine canal convergence profiles for the planning of the orifice-directed access approach in order to maintain maximal PCD, soffits, and trusses was previously described.3
Dynamic navigation (DN) uses data from a CBCT scan to guide the clinician during dental procedures. Overhead stereoscopic cameras track the position of markers attached to the dental handpiece and the patient’s jaw. The position of the instrument tip is shown overlaid over the patient’s virtual dentition on the system’s screen interface. As the clinician moves the instrument clinically, the virtual representation of the instrument moves on the screen, which provides real-time guidance.
Further details about the workflow of DN were described by Gambarini et al.4 Over the last few years, DN has been utilized mainly for the purpose of increasing the accuracy of dental implant placement. More recently, the potential benefits of DN in increasing the accuracy and efficiency of nonsurgical and surgical endodontic procedures were investigated.
For decades, the high magnification and illumination of the surgical operating microscope (SOM) has been an invaluable tool in different fields of medicine as well as in endodontics. Carr and Murgel5 discussed the benefits of the SOM in dentistry, which include improved ergonomics and photo documentation.
Over the years, the SOM used in different fields of medicine has evolved to incorporate HUD technology. In the 1990s, the first microscope with HUD capabilities was introduced to facilitate image-guided neurosurgery.6 HUD in the SOM involves the injection of a virtual image within the clinical field of view as seen through the binoculars.
Injected overlaid images include patient data such as pre-op imaging of interest or data from navigation systems. Incorporating a HUD is thought to improve ergonomics during medical surgeries as the clinician can visualize valuable information through the overlaid virtual image and directly visualize the surgical field itself.7 To the author’s knowledge, the clinical use of a HUD in an SOM in the field of dentistry has never been reported to date.
CASE REPORT
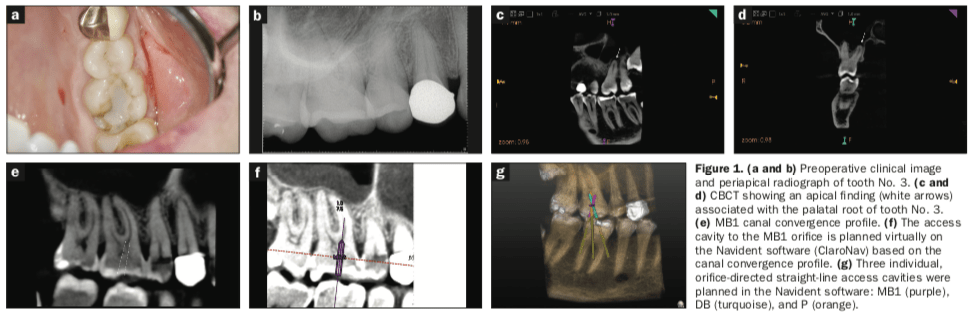
A 62-year-old female with a noncontributory medical history presented to the author’s practice with a chief complaint of pain to chewing in the upper right posterior area that began one month prior. Clinically, tooth No. 3 had an occlusal composite resin restoration demonstrating leakage and crack lines at the mesial and distal marginal ridges (Figure 1a). Two-dimensional radiographic interpretation revealed no compelling apical findings and no deep restoration, suggesting a coronal crack as the potential etiology (Figure 1b).
The patient was referred for a small field-of-view CBCT scan. Interpretation of the CBCT volume revealed a periapical finding located apical to the palatal root of tooth No. 3 (Figures 1c and 1d). Tooth No. 3 was diagnosed with pulp necrosis and symptomatic apical periodontitis. Treatment options were discussed, and the patient consented to nonsurgical endodontic treatment with DN guidance using the Navident system (ClaroNav).
In order to maintain as much PCD and pulpal roof dentin as possible, 3 separate access cavities were planned using the Navident software based on the canal convergence profiles: one to the distobuccal (DB) canal orifice, one to the palatal (P) canal orifice, and one to the mesiobuccal (MB1) canal orifice (Figures 1e to 1g).
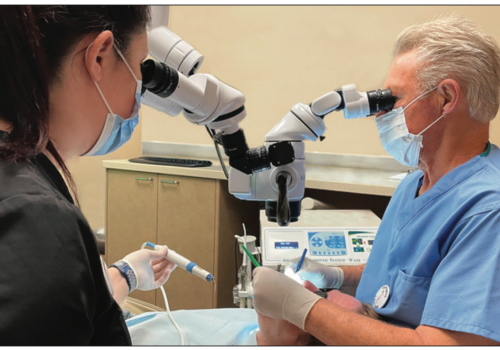
After local anesthesia, DN registration and calibration procedures were completed: After placement of the rubber dam isolation, the Navident fiducial marker was placed onto the maxilla, and the dentition was registered in order to merge the CBCT data to the actual clinical field.
The handpiece and bur were then calibrated as per Navident protocol. Access cavity preparation using DN was completed under the SOM with the simultaneous use of an experimental HUD prototype (Zumax Medical Co, Ltd) (Figures 2a and 2b) using a #859.31.010 needle tapered diamond bur (Brasseler USA).
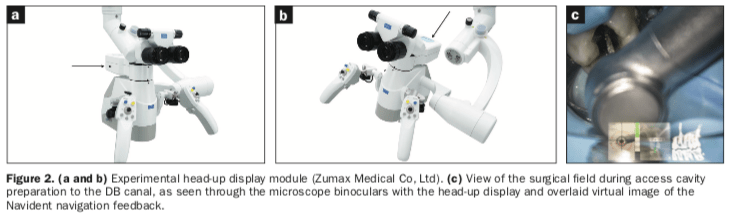
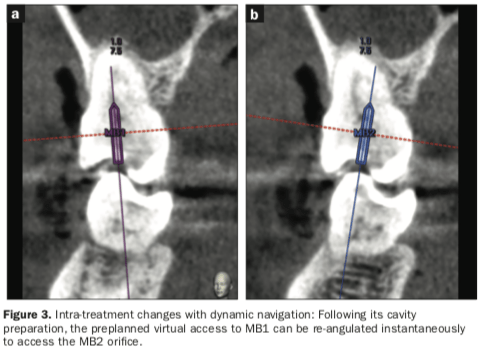
The access cavity preparation to the DB canal with simultaneous DN as viewed through the SOM binoculars is shown in Figure 2c. Following completion of straight-line access preparations to the MB1, DB, and P canals, the preplanned digital access path to the MB1 canal was re-angulated toward the MB2 canal (Figure 3).
The MB2 canal was then accessed in the same manner using DN and the SOM HUD. Glide paths and working lengths using the Ryder S3 electronic apex locator (MedicNRG) were obtained. Instrumentation to a size #20.06 in the buccal canals and #25.06 in the palatal canal was performed using the DCTaper rotary file system (SS White Dental). Disinfection was accomplished using full-strength (8%) sodium hypochlorite and the EndoActivator (Dentsply Sirona). The canals were dried with paper points and medicated with calcium hydroxide.
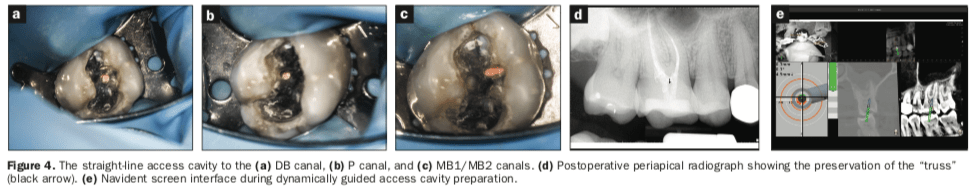
The access cavities were temporarily restored with Cavit Temporary Filling Material (3M) and a bonded composite resin restoration coronally. The patient returned 4 weeks later with a totally asymptomatic and functional tooth. The canals were re-accessed and obturated using warm vertical compaction of gutta-percha with AH Plus sealer (Dentsply Sirona). Figures 4a to 4c show the 3 separate orifice-directed access cavities with the maintenance of pulpal roof dentin. After the application of 37% acid etch, Futurabond DC (VOCO) and LuxaCore Z Dual (DMG America) were used to permanently restore the tooth.
A recommendation for an onlay indirect restoration was made. Figure 4d shows the postoperative radiograph, demonstrating the conservative access cavities, the preservation of the PCD, and the truss (black arrow).
DISCUSSION
Despite gaining interest from clinicians worldwide, minimally invasive approaches to endodontic access cavity preparation are still a subject of controversy. Conclusions from in vitro studies are often referenced to argue against minimally invasive endodontics.8 Unfortunately, the inherent limitations of these in vitro studies using mechanical-failure experiments lead to significant unreliability when translating results from benchtop experiments to clinical situations.9
Long-held concerns about the necessity of surgical invasiveness within the medical community decades ago have led to the eventual acceptance of minimally invasive therapy.10 Indeed, removal or manipulation of additional human tissue during various surgical procedures should be justified with demonstrable and compelling high-level evidence. In endodontics, the responsibility of demonstrating benefits should be on those who advocate for more dentin removal during access cavity preparation rather than those who advocate for less.
Randomized control trials are the source of the strongest evidence to guide clinicians in decision-making for different clinical interventions.11 Unfortunately, there are currently no such clinical studies available that suggest that minimally invasive approaches to access cavity preparation may offer an advantage over conventional ones or vice versa.
On the other hand, appraisal of the next best available evidence, which includes observational clinical studies with medium to long-term follow-up times, suggests otherwise.12-21 These studies consistently report that the main causes for extraction of ETT most often include recurrent caries or restorative, structural, or periodontal failures, with failures of true endodontic origin reported least frequently.
These findings suggest that DDC should be one of the main goals of endodontic treatment to achieve tooth longevity. As newer technologies have emerged, such as heat-treated rotary files, CBCT, DN, and improved irrigation and obturation methods, overcoming past limitations that have constrained the requirements for traditional access cavity designs is now possible.
The experimental HUD prototype used in this case report consisted of a display device projecting a mirror image of the Navident computer screen (Figure 4e) that was overlaid in real time directly into the clinical field of view as seen through the SOM using a custom-made optical module. Future applications of the HUD in the SOM involve the injection of apex locator reading data or pre-op imaging of interest and the overlay of virtual 3D models of dental structures using artificial intelligence and image processing.
The use of DN to obtain optimal DDC was also demonstrated in this case report. DN allowed for the planning and execution of ideally designed access cavities to maintain as much PCD and pulpal roof as possible while still achieving the biological objectives of endodontic treatment. Based on the canal convergence profiles, straight-line accesses were achieved individually to each canal orifice, which allowed for safe instrumentation using heat-treated NiTi rotary files.
Although the same procedural outcome can routinely be achieved by experienced clinicians using free-hand access cavity preparation, DN has the potential to streamline the process and significantly decrease treatment time and frustration.
Another approach to guided endodontics involves the use of 3D printed static guides. Potential limitations of static guides in endodontics include a lack of interocclusal distance to accommodate for longer drills, the inability to use high-speed drills or burs, and the wait time involved with the fabrication of the static guide(s), which complicates same-day endodontic treatment. The need for the fabrication of multiple guides in order to access all canals in multi-rooted teeth and the inability to easily change the treatment plan during the guided procedure in light of new clinical information or challenges are also limiting factors.22
These limitations are usually not present with DN. Indeed, an access cavity can be planned within a few minutes, making DN ideal for emergency treatment. The Navident system can be used with many different instrument tips, including high-speed and low-speed burs, ultrasonic tips, or piezo surgical tips. Furthermore, DN allows for instantaneous changes to the treatment plan during the procedure (Figure 3).
The accuracy of DN for endodontic procedures has never been evaluated in vivo. Compiled results from in vitro studies in the fields of implantology and endodontics, as well as results from in vivo studies in implantology, suggest that DN currently has an error range of approximately 0.5 to 1 mm.23-29
Although DN offers a tremendous benefit in increasing the accuracy of different clinical procedures, its current error range remains high for endodontic applications. Furthermore, the current workflow for DN, as recommended by manufacturing companies, involves the total commitment of the clinician’s attention to the system’s computer screen interface, which is positioned away from the surgical field.
As the clinician commits full attention to DN during access cavity preparation, other valuable information, such as direct visual feedback from the dentin map, cannot be accessed. With a HUD, the clinician can use DN as an adjunct without having to look away from the surgical field. This freedom allows the clinician to selectively focus on different valuable pieces of information without any compromise and may help increase the accuracy of access cavity preparations with optimal DDC as opposed to using DN alone.
Furthermore, having to look up at a computer screen during DN procedures may feel unnatural to the clinician who is used to looking at the surgical field through the SOM.
Combining the benefits of both DN and SOM simultaneously also allows for minimizing wasted movements and time as well as maximizing optimal ergonomics.
CONCLUSION
This case report demonstrated the potential of DN to prepare endo-dontic access cavities efficiently and safely with maximal DDC. The SOM HUD further demonstrated enhanced ergonomics. Future clinical studies are required to determine the accuracy of DN for endodontic access cavity preparations.
ACKNOWLEDGEMENTS
The author would like to thank Drs. Viraj Vora and Dale Jung for their help with reviewing the manuscript.
REFERENCES
1. Clark D, Khademi J. Modern molar endodontic access and directed dentin conservation. Dent Clin North Am. 2010;54(2):249–73. doi:10.1016/j.cden.2010.01.001
2. Clark D, Khademi J, Herbranson E. The new science of strong endo teeth. Dent Today. 2013;32(4):112, 114, 116–7.
3. Schwartz R, Canakapalli V. Best practices in
endodontics: A desk reference. Quintessence Publishing Co, Inc; 2015.
4. Gambarini G, Galli M, Stefanelli LV, et al. Endodontic microsurgery using dynamic navigation system: a case report. J Endod. 2019;45(11):1397-1402.e6. doi:10.1016/j.joen.2019.07.010
5. Carr GB, Murgel CA. The use of the operating microscope in endodontics. Dent Clin North Am. 2010;54(2):191-214. doi:10.1016/j.cden.2010.01.002
6. Friets EM, Strohbehn JW, Hatch JF, et al. A frameless stereotaxic operating microscope for neurosurgery. IEEE Trans Biomed Eng. 1989;36(6):608–17. doi:10.1109/10.29455
7. Ma L, Fei B. Comprehensive review of surgical microscopes: technology development and medical applications. J Biomed Opt. 2021;26(1):010901. doi:10.1117/1.JBO.26.1.010901
8. Silva EJNL, Versiani MA, Souza EM, et al. Minimally invasive access cavities: does size really matter? Int Endod J. 2021;54(2):153-155. doi:10.1111/iej.13462
9. Ordinola-Zapata R, Fok ASL. Research that matters: debunking the myth of the “fracture resistance” of root filled teeth. Int Endod J. 2021;54(3):297-300. doi:10.1111/iej.13479
10. Frampton S, Kneebone RL. John Wickham’s new surgery: ‘minimally invasive therapy’, innovation, and approaches to medical practice in twentieth-century Britain. Soc Hist Med. 2017;30(3):544-566. doi:10.1093/shm/hkw074
11. Brignardello-Petersen R, Carrasco-Labra A, Glick M, et al. A practical approach to evidence-based dentistry: III: how to appraise and use an article about therapy. J Am Dent Assoc. 2015;146(1):42-49.e1. doi:10.1016/j.adaj.2014.11.010
12. Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of non-surgical root canal treatment: part 2: tooth survival. Int Endod J. 2011;44(7):610–25. doi:10.1111/j.1365-2591.2011.01873.x
13. Landys Borén D, Jonasson P, Kvist T. Long-term survival of endodontically treated teeth at a public dental specialist clinic. J Endod. 2015;41(2):176–81. doi:10.1016/j.joen.2014.10.002
14. Stavropoulou AF, Koidis PT. A systematic review of single crowns on endodontically treated teeth. J Dent. 2007;35(10):761–7. doi:10.1016/j.jdent.2007.07.004
15. Lynch CD, Burke FM, Ní Ríordáin R, et al. The influence of coronal restoration type on the survival of endodontically treated teeth. Eur J Prosthodont Restor Dent. 2004;12(4):171–6.
16. Petersson K, Fransson H, Wolf E, et al. Twenty-year follow-up of root filled teeth in a Swedish population receiving high-cost dental care. Int Endod J. 2016;49(7):636–45. doi:10.1111/iej.12495
17. Vire DE. Failure of endodontically treated teeth: classification and evaluation. J Endod. 1991;17(7):338–42. doi:10.1016/S0099-2399(06)81702-4
18. Sjogren U, Hagglund B, Sundqvist G, et al. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16(10):498-504. doi:10.1016/S0099-2399(07)80180-4
19. Fonzar F, Fonzar A, Buttolo P, et al. The prognosis of root canal therapy: a 10-year retrospective cohort study on 411 patients with 1175 endodontically treated teeth. Eur J Oral Implantol. 2009;2(3):201–8. https://pubmed.ncbi.nlm.nih.gov/20467630/
20. Lee AH, Cheung GS, Wong MC. Long-term outcome of primary non-surgical root canal treatment. Clin Oral Investig. 2012;16(6):1607–17. doi:10.1007/s00784-011-0664-2
21. Olcay K, Ataoglu H, Belli S. Evaluation of related factors in the failure of endodontically treated teeth: a cross-sectional study. J Endod. 2018;44(1):38-45. doi:10.1016/j.joen.2017.08.029
22. Buchanan LS, Maupin C, Khademi J. A revolutionary protocol for endodontic access. Dent Today. 2018.
23. Jorba-García A, González-Barnadas A, Camps-Font O, et al. Accuracy assessment of dynamic computer-aided implant placement: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(5):2479-2494. doi:10.1007/s00784-021-03833-8
24. Jain SD, Saunders MW, Carrico CK, et al. Dynamically navigated versus freehand access cavity preparation: a comparative study on substance loss using simulated calcified canals. J Endod. 2020;S0099-2399(20)30578-1. doi:10.1016/j.joen.2020.07.032
25. Dianat O, Nosrat A, Tordik PA, et al. Accuracy and efficiency of a dynamic navigation system for locating calcified canals. J Endod. 2020;S0099-2399(20)30501-X. doi:10.1016/j.joen.2020.07.014
26. Gambarini G, Galli M, Morese A, et al. Precision of dynamic navigation to perform endodontic ultraconservative access cavities: a preliminary in vitro analysis. J Endod. 2020;46(9):1286-1290. doi:10.1016/j.joen.2020.05.022
27. Jain SD, Carrico CK, Bermanis I. 3-Dimensional accuracy of dynamic navigation technology in locating calcified canals. J Endod. 2020;46(6):839-845. doi:10.1016/j.joen.2020.03.014
28. Zubizarreta-Macho Á, Muñoz AP, Deglow ER, et al. Accuracy of computer-aided dynamic navigation compared to computer-aided static procedure for endodontic access cavities: an in vitro study. J Clin Med. 2020;9(1):129. doi:10.3390/jcm9010129
29. Chong BS, Dhesi M, Makdissi J. Computer-aided dynamic navigation: a novel method for guided endodontics. Quintessence Int. 2019;50(3):196-202. doi:10.3290/j.qi.a41921
ABOUT THE AUTHOR
Dr. Nadeau graduated with a DDS degree from Dalhousie University and earned a Master’s of Science in endodontics from the University of Toronto. He has developed particular interests in dynamic navigation for endodontics, ergonomics in microscope dentistry, restoratively driven endodontics, and clinical decision-making. Dr. Nadeau is in full-time private practice in Kingston, Ont, Canada. He can be reached at bnadeau29@gmail.com.