There has been an explosion in the various specialties using cosmetic botulinum neurotoxins, in particular botulinum neurotoxin type A (BTX-A) or BOTOX (Allergan). There were 3,181,592 BTX-A cosmetic treatments administered in the United States in 2006, there are 3,000 publications on BTX-A in scientific and medical journals, and 100 years of study in botulinum neurotoxins.1 BTX-A cosmetic ranks No. 1 on list of “Top 5 Surgical and Nonsurgical Physician Administered Cosmetic Procedures.”2 In addition BTX-A cosmetic procedures are proving to be a great adjunct to cosmetic dentistry.3,4 BTX-A, when used for treating oral rhytids, is the most common new area for which patients request treatment.5 The incorporation of botulinum toxin into cosmetic dentistry offers a complete and comprehensive cosmetic treatment plan for patients. Dentists are able to supply the patient with a sound differential diagnosis, which may include dental, orthodontic, orthognathic, soft-tissue, or a combination of these treatment modalities to arrive at a superior anterior lip and perioral cosmetic result.6
This article presents a case of lip augmentation using BTX-A. Select injection points were used to administer BTX-A using the Gordon Classification Method. The goal of the treatment was to reduce the severity of the lip rhytids. The patient’s rhytids were reduced from a 3 (fine wrinkles present at rest, deeper lines with facial expression) to a 0 to 1 (absent to mild) after one week post-injection treatment. The rhytids were evaluated pretreatment and post-treatment using the Kim Rated Numeric Kinetic Line Scale (KRNKLS) and the Carruthers Wrinkle Severity Rating Scale (CWSRS).
PERIORAL INJECTIONS
BTX-A has been proven to reduce the perioral lines around the lips. Injecting adjacent to the fine lines around the mouth results in a smoother appearance of the lips and an eversion of the vermilion border of the lips.7,8 It is important to understand that the perioral lines around the mouth have both a static and dynamic relationship, which is different than the lines and folds of the upper face. The upper face lines and folds manifest themselves in the kinetic motion predominantly.
There are ultimately 3 goals in perioral BTX-A injections:
Goal No. 1—The removal of kinetic rhytids. This is done by having the patient contract the lips in a pursing movement and observing the rhytids. If the rhytids deepen and coincide with the contraction of the mouth, then they are most likely due to the hyperkinetic action of the orbicularis and can be treated with BTX-A therapy. If the rhytids do not correspond or deepen when the patient purses their lips, these lines are a manifestation of loss of lip volume and would be better suited for injectable filler therapy.
Goal No. 2—To achieve an increase in lip surface area. An increase in the lip surface area is acquired by releasing the constrictive force of the orbicularis oris, whereby reversing the effect of the constriction of the lip at the zone A.
Goal No. 3—To establish a desired eversion of the lip. Eversion of the vermilion border of the lips can be acquired by precise injection of BTX-A in the perioral area (zone A). By weakening the superficial orbicularis oris, there is an eversion of the G to K line angle and a corresponding increase in surface area of the lips proper (zone B).
PROCEDURE
Materials
BTX-A is supplied in a vial containing 100 U of vacuum-dried neurotoxin complex. The recommended reconstitution is 2.5 mL of 0.9% nonpreserved saline to final concentration of 4.0 U/0.1 mL.9 Preserved saline can be used to reconstitute a BTX-A vial. The advantage to using preserved saline is diminished pain upon injection of BTX-A, up to 54% with 0.09% preserved benzyl alcohol. The results of a study showed that 100% of the patients injected with preserved isotonic saline reported less pain than when injected with nonpreserved isotonic saline (P < .001).10 The main objective in the dilution ratio of the BTX-A is to allow effective control of the administering dose.11 In addition, due to the dense musculature of the orbicularis oris, injecting larger volume units causes unnecessary pain. The usual reconstitution ratio for the perioral region is 1.0 mL to 2.5 mL nonpreserved saline per vial of 100 U of BTX-A.12 This dilution ratio gives a dispersion area of 1 to 1.5 cm, so this is the minimum spacing of injections.
Prescribing information included in BTX-A suggests that reconstituted BTX-A should be used within 4 hours.13 Clinical studies indicate that a reconstituted BTX-A solution with nonpreserved saline maintains its efficacy up to 4 to 6 weeks before use, when stored at 4°C.14,15 Recent studies suggest that there is no significant difference in BTX-A stored in a refrigerator versus a freezer.16
 |
 |
|
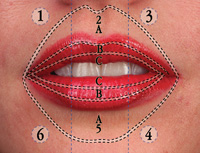
Figure 1. Pictured here is the Gordon classification for the lip and perioral area. This classification method maps out the area to be augmented into zones and segments. There are 6 segments and zones A, B, and C. So the exact middle point in the lower lip would be located at segment 5 zone B. |
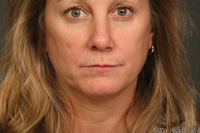
Figure 2. This patient’s rhytids are classified as a 3 using (Table 1) the Carruthers Wrinkle Severity Rating Scale and a 3 using (Table 2) the Kim Rated numeric kinetic line scale. |
Immunogenicity
The development of antibodies is a concern for the use of botulinum toxin.18 When botulinum toxin resistance has been reported, it has been less than 5%.19 Factors that potentially contribute to botulinum resistance include: dose and frequency of treatment intervals.20 Patients treated with high doses (300 units of BTX-A or higher) at frequent intervals potentiate the development of antibodies. The total dosage for lip and perioral augmentation usually ranges between 6 to 20 units, which is significantly below 300 unit or higher value. The frequency of injection should be spaced out several months between treatments.
It is not uncommon to provide additional injections one week post-initial treatment, when the desired effect has not been met, or for symmetrical balancing purposes. It is recommended to limit the total amount of toxin to less than 100 U per session and to avoid booster injections for a minimum of 3 months.21 If additional injections are required for a patient’s postinitial treatment, it should be noted in his or her chart. The sequential treatments at least 3 months later with the adjusted injection dosages should provide an adequate cosmetic result with the initial treatment.
CASE REPORT
The case presented below is a 45-year-old female who presented for a consultation on conventional aesthetic reconstruction (Figure 2). One of her chief complaints was the continued development of lines around her mouth. She states that they are continually getting “worse.” The patient also commented on the loss of maxillary lip volume, compared to when she was younger.
| Table 1. Carruthers Wrinkle Severity Rating Scale (1 to 5) |
|
 |
 |
|
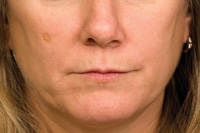
Figures 3 and 4. Evaluating the patients rhytids in kinetic as well as relaxed position is paramount in determining if botulinum neurotoxin type A (BTX-A) will be beneficial. If the lines coincide in contraction of the obicularis oris to when the lips are relaxed then these are lines can be potentially relieved with BTX-A treatment. |
| Table 2. Kim Rated Numeric Kinetic Line Scale (0 to 4) |
| 0. No Wrinkles. 1. Wrinkles not presentat rest, fine. 2. Wrinkles not present at rest, deep lines with facial expression. 3. Fine wrinkles present at rest, deeper lines with facial expression. 4. Deep wrinkles at rest, deep furrows with facial expression. |
 |
 |
|
Figure 5. Pictured here is the diabetic injection syringe (3/10 cc, 8 mm needle length, 31-gauge ultrafine needle). This is the optimal injection syringe for lip BTX-A treatment. |
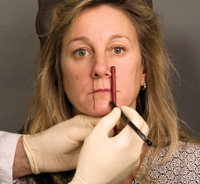
Figure 6. Pictured here is the marking on the patient’s lips and perioral area. Using a generic makeup applicator and placing targeted spots around the lips will guide the augmenter during their treatment. |
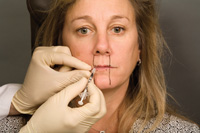 |
| Figure 7. Simply placing the needle between the fingers and slightly moving the lips during injection can relieve a significant amount of discomfort during injection. This is why it is important to mark the intended locations of injection before beginning treatment. |
Lines were drawn downward from the corners of the nose (puriform recess) with a cosmetic pencil (Figure 6) defining the borders within which the placement of BTX-A will not compromise lip competency. Anywhere lateral to these defined borders may affect lip competency.
With the same cosmetic pencil, locations were selected around the vermilion border in zone A for injection of BTX-A. One can try and place an injection point directly in the fissure of a lip rhytid, but that is not necessary since the BTX-A will spread at the dilution ration we have here the diameter of an eraser head of a No. 2 pencil or 1 cm. Rather the injector should concentrate on symmetrical placement of the injection points around the mouth.
Select locations for the application of BTX-A were marked around the lips in zone A. Zone A is the only area where BTX-A is applied, neither zone B or C will receive BTX-A treatment. One unit was placed in each of the marked spots around the lips with BTX-A. Since no anesthetic is used there is a slight pinch with each injection. This discomfort can be minimized by distending the lips with your index finger and thumb during the injection, similar to the Gates method of distraction for conventional dental injections (Figure 7). The injection depth should be half the depth of our selected needle length or 4 mm depth.
Post-Injection Recall
The process of the botulinum molecule binding and interfering with nerve transmission takes 6 to 36 hours after initial injection.25,26 The duration of effect to restore functional muscle activity takes 3 to 6 months.27 I have patients return for recall evaluation one week after the initial treatment. At this point, I am able to assess the result of the lip and perioral BTX-A treatment. If the desired effect is not achieved, I may apply another unit in each previously selected location.
 |
 |
|
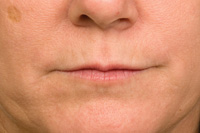
Figure 8. This is one-week post injection with BTX-A. Notice the diminished lines upon static position of lips. Now we can evaluate if additional dermal fillers can be used to optimize augmentation. |
Figure 9. This is 2-week post-op picture with the addition of dermal filler injections in the lips. |
One can continue on with filler augmentation if it is felt that there can be an additional cosmetic benefit. In this case, the decision was made to continue with a cross-linked hyaluronic acid filler injections.
| Table 3. Wrinkle Improvement Scale (0 to 3) |
| 0. No improvement. 1. Mild improvement. 2. Moderate improvement. 3. Significant improvement. |
SUMMARY
BTX-A cosmetic injections around the lip and perioral area take approximately 20 minutes of clinical time to perform. The procedure is a minimally invasive way to provide substantial cosmetic improvement to the signs of aging around the lips and can provide an adjunct to lip augmentation. Incorporating this new treatment into your clinical practice requires additional training. It is best to attend training on both BTX-A injectable fillers for a complete education. (There a many more techniques and injection locations for different lip and perioral cosmetic effects.)
The above case and dosages demonstrated a conservative approach to achieve a superior lip and perioral result.
References
- Botox Cosmetic and Botox (Botulinum Toxin Type A) By the Numbers. Allergan Press Release. 2008.
- Top 5 surgical and nonsurgical cosmetic procedures. American Society for Aesthetic Plastic Surgery Web site. http://www.surgery.org/download/2006-Top5.pdf. Accessed March 17, 2009.
- Niamtu J III. Cosmetic oral and maxillofacial surgery: the frame for cosmetic dentistry. Dent Today. Apr 2001;20:88-91.
- Niamtu J III. Aesthetic uses of botulinum toxin A. J Oral Maxillofac Surg. 1999;57:1228-1233.
- Fagien S. Botox for the treatment of dynamic and hyperkinetic facial lines and furrows: adjunctive use in facial aesthetic surgery. Plast Reconstr Surg. 1999;103:701-713.
- Gordon RW. Vermilion Dollar Lips, Lip and Perioral Augmentation for the Cosmetic Dentist. Vermilion Dollar Publications; 2008.
- Fagien S. Extended use of botulinum toxin type A in facial aesthetic surgery. Aesthetic Surg J. 1998;18:215-219.
- Foster JA, Wulc AE. The use of botulinum A toxin to ameliorate facial dynamic lines. Int J Aesthet Restor Surg. 1996;4:137-144.
- Botox Cosmetic [package insert]. Irvine, CA: Allergan, Inc; 2008.
- Alam M, Dover JS, Arndt KA. Pain associated with injection of botulinum A exotoxin reconstituted using isotonic sodium chloride with and without preservative: a double-blind, randomized controlled trial. Arch Dermatol. 2002;138:510-514.
- Sposito MM. New indications for botulinum toxin type A in cosmetics: mouth and neck. Plast Reconstr Surg. 2002;110:601-611.
- Benedetto AV. The cosmetic uses of botulinum toxin type A. Int J Dermatol. 1999;38:641-655.
- BOTOX [package insert]. Irving, CA: Allergan; 2008.
- Hexsel DM, De Almeida AT, Rutowitsch M, et al. Multicenter, double-blind study of the efficacy of injections with botulinum toxin type A reconstituted up to six consecutive weeks before application. Dermatol Surg. 2003;29:523-529.
- Garcia A, Fulton JE Jr. Cosmetic denervation of the muscles of facial expression with botulinum toxin: a dose-response study. Dermatol Surg. 1996;22:39-43.
- Carruthers A, Carruthers J. Procedures in Cosmetic Dermatology: Botulinum Toxin. Philadelphia, PA: Saunders; 2005.
- Carruthers J, Fagien S, Matarasso SL, et al. Consensus recommendations on the use of botulinum toxin type A in facial aesthetics. Plast Reconstr Surg. 2004;114(suppl):1S-22S.
- Jankovic J, Schwartz K. Response and immunoresistance to botulinum toxin injections. Neurology. 1995;45:1743-1746.
- Ludlow CL, Hallett M, Rhew K, et al. Therapeutic use of type F botulinum toxin. N Engl J Med. 1992;326:349-350.
- Sankhla C, Jankovic J, Duane D. Variability of the immunologic and clinical response in dystonic patients immunoresistant to botulinum toxin injections. Mov Disord. 1998;13:150-154.
- Matarasso SL. Complications of botulinum A exotoxin for hyperfunctional lines. Dermatol Surg. 1998;24:1249-1254.
- Carruthers A, Carey W, De Lorenzi C, et al. Randomized, double-blind comparison of the efficacy of two hyaluronic acid derivatives, restylane perlane and hylaform, in the treatment of nasolabial folds. Dermatol Surg. 2005;31:1591-1598.
- Kim EJ, Reeck JB, Maas CS. A validated rating scale for hyperkinetic facial lines. Arch Facial Plast Surg. 2004;6:253-256.
- Kim EJ, Ramirez AL, Reeck JB, et al. The role of botulinum toxin type B (Myobloc) in the treatment of hyperkinetic facial lines. Plast Reconstr Surg. 2003;112(suppl):88S-93S.
- Carruthers A, Carruthers J. Use of botulinum toxin A for facial enhancement. In: Klein AW, ed. Tissue Augmentation in Clinical Practice. 2nd ed. New York, NY: Taylor & Francis Group; 2006.
- Meunier FA, Schiavo G, Molgo J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J Physiol Paris. 2002;96:105-113.
- Humeau Y, Doussau F, Grant NJ, et al. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie. 2000;82:427-446.
Dr. Gordon graduated from Marquette University School of Dentistry and continued his education in a residency program in periodontics, as well as additional oral/surgery training. He practices in Clearwater Florida and lectures often, instructing oral/facial augmenters on his classification, diagnostic and reconstructive lip and perioral augmentation techniques. He can be reached at dr.g@vermiliondollarlips.com or go online at vermiliondollarlips.com to order his book, instructional videos, and learning seminars.
Disclosure: Dr. Gordon reports no conflict of interest.