The US Food and Drug Administration (FDA) has cleared Nobio’s Infinix Flowable Composite and Bulk Fill Flow Composite for marketing. According to the company, they are the first products cleared by the FDA to incorporate Nobio’s patented QASi particle technology for maintaining restoration integrity and protecting against degradation by bacteria over time. Nobio’s Infinix Universal Composite and Universal Bond, which also feature QASi technology, are pending FDA clearance.
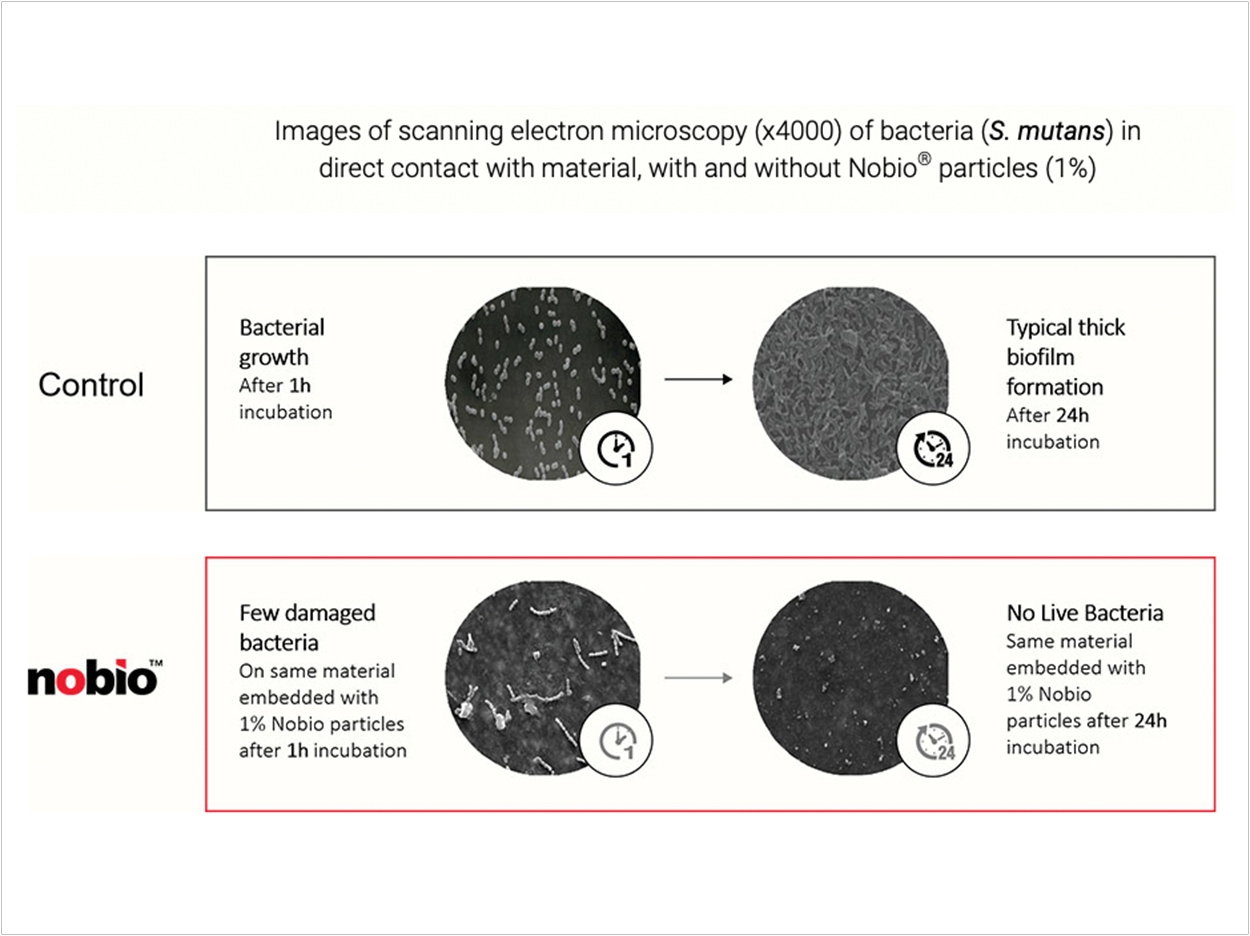
QASi particles, comprising quaternary ammonium silica-dioxide, combine a high concentration of antimicrobial molecules that are covalently bound to a solid core to form an insoluble, potent, long-lasting antimicrobial structure, Nobio says. The antimicrobial effect occurs only when bacteria contact the material containing these particles, offering significant advantages versus traditional approaches that rely on the release of antimicrobial molecules, which eventually deplete and also may affect normal flora, the company reports.
Plus, the filling material retains these insoluble particles following polymerization, producing a long-term effect, Nobio says. Long-term antimicrobial protection and a stable molecular structure are especially important in dental restorations, which are intended to remain in the oral environment for decades, according to the company. More than 200 million restorations are performed in the United States each year, many of them to replace previous restorations that have failed, at an annual cost of more than $5 billion, Nobio says.
Related Articles
Plaque and Microbes Defeated by New Dental Material
Self-Assembling Antimicrobial Materials Prevent Recurrent Caries
Injectable Nanoparticles May Guard Teeth Against Tooth Decay












