 |
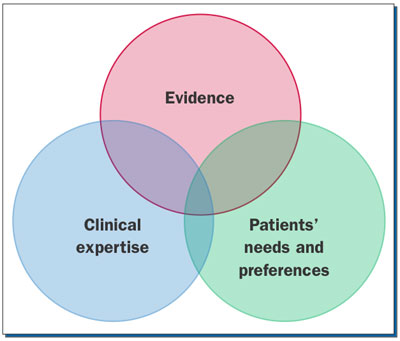
| Figure. Evidence-based dentistry is right in the center, where circles overlap.5 |
Do you apply the “80-20 rule” at work? The 80-20 rule, or Pareto principle,1 is the idea that a relatively small percentage of any cause (20%) creates most of the subsequent effects (80%). In a business context, this means that 80% of a company’s business stems from 20% of its customers.
In dentistry, teeth make up only 20% of the surface area of the mouth, with the other 80% including interproximal spaces, the dorsum of the tongue and cheeks, and below the gumline; all reservoirs and niches for biofilm.2 There is a similar 80-20 situation happening with biofilm: 20% are pathogens and 80% is a slime layer composed of self-secreted glycoproteins and polysaccharides that is difficult to penetrate.3 Brushing and interproximal cleaning alone is not enough to disrupt biofilm. With a new-generation rinse now available, it’s time to revisit recommendations, as our case will illustrate.
CASE: FORLORN AT COLLEGE
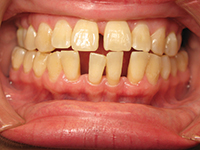
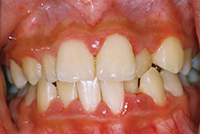
Ashley is a 21-year-old young woman who is new to the practice. She’s attending the nearby university, studying bioscience. She reports using antihistamines only as needed and vitamins daily. She stated in her review of systems that she is consuming quite a few energy drinks to help her get her homework done. A friend told her about adding vodka and making “vod-bombs” (on the weekends). She noticed her gums have been bleeding and she has some white spots on her teeth. She also mentioned that she wakes up many mornings with cotton mouth. She is nervous because her dental health has always been good; she never had a cavity. She still brushes and flosses the same as she has since childhood, but she thinks her parents are going to be very disappointed if she ends up with teeth and gum problems.
Ashley is concerned about the changes she is noticing in her mouth. This is a good first step for the clinician to intervene. She continues to follow the home care regimen she was taught as a child, but circumstances have changed. She is showing signs and symptoms of early breakdown and is now at high risk for dental disease, yet she is at a stage where reversal and healing can occur. This is where minimally invasive dentistry works the best. What kind of plan should be set up for Ashley? How can her early disease be reversed? How do you communicate a home care regimen with Ashley without making her feel you are treating her like a child?
BIOFILM: IT’S NOT JUST PLAQUE/CALCULUS!
Often in practice, periodontal disease is synonymous with periodontitis. This is incorrect. Periodontal disease is a continuum, a type of wound. Mealey and Rose4 discuss the periodontium as a unique ecological niche in the human body. Prior to the eruption of teeth, the tissue is intact, yet inhabited by bacterial communities that don’t challenge the individual’s health, similar to the bacteria that thrive harmlessly on your skin. When the teeth erupt, this surface can have as many as 32 objects violating this formerly intact mucosa.4 This creates the potential for biofilm and promoters of inflammation to reach the bloodstream.
Often in practice, the words “biofilm” and “plaque” are used interchangeably. This too can be incorrect, or at least confusing. When talking about home care, we need to consider the makeup and location of biofilm, not plaque. Many of us completed our education at a time when bacteria were studied in a free-floating planktonic state. This led to the concept that certain pathologic pathogens were the reason for the breakdown.
|
Dental professionals once thought that the thorough removal of supra- and subgingival plaque and deposits, and proper home care, would promote health. It is now known that biofilms are medically/dentally important. We also know that microbes living in a planktonic state that are nonadherent and free-floating cause few diseases. An oral biofilm environment is an accumulation of a mixed population of bacteria, fungi, or protozoa that produce large amounts of slime or matrix material around themselves.4 Using the terms “biofilm” and “plaque” interchangeably can confuse our understanding.
Because of the work of Costerton,3 we know that biofilm is a complex community and it has a tremendous ability for self-preservation.3 Yet biofilm is not inherently bad. Biofilms are common in nature, yet damage can occur. Biofilm moving from a healthy to disease-inducing state can be prevented by routine home care. It is the routine disruption that keeps the biofilm in a healthy state.
With this understanding of the 80-20 of biofilm, let’s look again at the 80-20 of common oral home care. Emphasizing brushing and interproximal cleaning to disrupt the biofilm is not enough. mouthrinse must be added to penetrate the 80% slime layer adhering to 80% of the oral cavity.
Using Evidence-Based Dentistry in Product Selection
How can professionals make a recommendation to Ashley? The answer lies in evidence-based dentistry (EBD). EBD is research. Clinical recommendations have 3 parts: (1) the evidence, (2) our professional experience and judgment, and (3) the patient’s clinical/social circumstances and preferences (Figure).5 Clinical recommendations are the overlap of these 3 areas.
In the 21st century, EBD can now be accessed easily via the Internet. Computers have become ubiquitous; most households have at least one or more of them. The reason for this is that we can do/learn/create differently with them. Using computers in healthcare, including the soon-to-be interoperable electronic health records, doesn’t just take what we have done traditionally and make it electronic. Rather, the use of computers opens options we didn’t have before, including finding research as part of the EBD process. The ADA has developed a website dedicated to EBD at ebd.ada.org.
Although EBD is not a linear process, we will break down our journey into steps. EBD requires the judicious integration of systematic assessments of clinically relevant scientific evidence. We will begin step one in our EBD journey of mouthrinses by looking at 5 antimicrobial active ingredients in many of the over-the-counter (OTC)/prescription mouthrinse options.
Evidence-Based Dentistry, Step 1—Science of the Options
There are 5 main antimicrobial active ingredients in mouthrinses on the market today (Table).
Essential oils have been around for thousands of years. They were first added to LISTERINE in 1879, but used as an antiseptic, not a mouthwash. In 1895, dentists started using it. In 1914, it became the first OTC mouthwash. LISTERINE is probably the most researched mouthwash on the market. The 4 essential oils used in the mouthwash are thymol, menthol, eucalyptol, and methyl salicylate. The oils disrupt the bacterial cell wall and kill biofilm and gingivitis organisms rapidly and nonselectively. Essential oils exhibit a broad spectrum of activity against Gram-positive and -negative bacteria, as well as fungi.
As a result of reducing the number of pathogenic bacteria in the mouth, biofilm endotoxin levels are also reduced. This in turn decreases the pathogenicity of biofilm and the development of gingivitis.6,7 Essential oil rinses are available in both alcohol-containing and alcohol-free versions; however, the alcohol-free products might not meet the same efficacy standards. The alcohol in therapeutic mouthrinses contains pharmaceutical-grade denatured alcohol to solubilize all the ingredients.
As with essential oils, chlorhexidine (CHX) also ruptures the bacterial cell membrane, leading to rapid leakage of cell contents and cell death. Unlike essential oils, CHX binds salivary mucins, which reduces pellicle formation and in turn inhibits biofilm bacteria colonization. CHX also binds bacteria, further inhibiting their absorption onto tooth surfaces. CHX exhibits a broad spectrum of antimicrobial activity and is effective against both Gram-positive and -negative bacteria.8-9 CHX is available with and without alcohol. Due to its substantivity, CHX is often called the gold standard to which other rinses are compared.
Cetylpyridinium chloride (CPC) is similar to CHX in that it too ruptures the bacterial cell membrane, leading to rapid leakage of cell contents and cell death. CPC may also alter bacterial metabolism and inhibit cell growth. CPC was first discussed in the scientific literature in the late 1930s and is included in many popular mouthrinses with varying concentrations, the most popular being Crest Pro-Health. It is important to recognize that the minimum recognized therapeutic concentration for CPC is 0.045%. Many cosmetic mouthrinses contain CPC at concentrations below 0.045%, and do not provide antiplaque/antigingivitis benefits. There are both alcohol-containing and alcohol-free products on the market.10,11
Stabilized chlorine dioxide (CloSYS, Oxifresh) has been around for almost 200 years, but there are insufficient studies showing clinical efficacy against gingivitis. It also does not have the ADA seal. Manufacturers claim the products oxidize, causing chemicals to unite with oxygen and kill bacteria that cannot survive in an oxygenated environment. There is a body of literature to show that chlorine dioxide reduces oral malodor. One in vitro study shows the potential for microbial kill. This study states that it is “an attractive option to induce compliance in patients concerned with taste and discoloration.”
The advantage of using rinses with chlorine dioxide is that they do not contain alcohol, they do not cause staining, they are pH-balanced, and can be used with or without flavorings. They do not require a prescription. However, more long-term research needs to be done.12,13
There is a new ingredient on the US market that shows great promise: Delmopinol hydrochloride 0.2% in G·U·M PerioShield (Sunstar Americas) is a proprietary key ingredient that creates a less-adhesive environment for bacteria and biofilm. This rinse prevents bacteria from sticking to the teeth, forming an invisible protective shield over the teeth and gingiva that bacteria cannot penetrate, essentially reducing biofilm buildup. The product breaks down the biofilm and bacteria, making them easier to remove, while coating the teeth and gums to prevent additional biofilm from sticking. As a result, continual use helps maintain a healthy, balanced microflora. In other words, delmopinol does not directly kill anything. There is a small amount of alcohol (1.5%). It is less staining than CHX and has shown good results in clinical trials.14-17
The product has been available in Europe for at least 10 years, and just recently came to the US market. This rinse was studied in meta-analyses of 8 double-blind studies looking at it as an adjunct to gingival health and biofilm control measures. Delmopinol met the efficacy criteria of the ADA in studies of extended durations. According to the manufacturer, G·U·M PerioShield was developed for patients with chronic gum inflammation, gingivitis, and severe biofilm buildup. It has been proven safe and effective for long-term use. The product is currently the only oral rinse available approved by the FDA as a device (Class II medical device), whereas all other antibiofilm/antigingivitis rinses are classified as drugs. An FDA Class II medical device is defined as: “…intended to affect the structure or any function of the body of man or other animals, and which does not achieve any of its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.”18
This approval clearly shows the difference in the mechanism of action.
Now we have the science for our EBD process; next is our professional expertise.
| The Single Implant Crown | |||||||||||||||||||||||
|
Tom M. Limoli Jr Don’t get me wrong. I have no problem with monitoring, focusing, and improving your overall practice profitability. In the absence of both profit as well as a sustained profit margin, we would all be out of business. I do have a problem with those who preach the concept of keeping the cart in front of the horse when, in fact, there was no problem with the original configuration. If the cart is broken, fix it. If the horse is tired, rest it. Rearranging the same parts in a similar yet reversed sequence simply changes the order of the outcome. You may get to the same place, but what did it cost you to get there? Is your overhead increased? Are your supply costs increased? Are your administrative costs increased? Again, if it isn’t broke, don’t fix it. After all, you just may discover that the best solution has always been right before your eyes. There is nothing mysterious about treating the early stages of periodontal disease in a nonsurgical manner, provided the process is started with an accurate as well as conclusive documented diagnosis. The American Academy of Periodontology specifies the 15 subcomponents of the evaluation process in their “parameters of care.” Probing depths, location of the gingival margin (clinical attachment level), and the presence of bleeding on probing is only one of the 15 subcomponents (perio.org). Documentation of oral hygiene instructions (OHI) includes the method of instruction used (audio, audio-visual, hands-on, etc), the duration of the visit, and the contents specific to the clinical condition of the patient as dictated by the treatment plan. Most reimbursement contracts limit OHI to medically or physically compromised patients. Many contracts reimburse in conjunction with periodontal surgery and comprehensive orthodontic treatment. The dispensing of certain drugs and or medicaments is again becoming more common in the dental office. Health plan controlled “mail order” pharmacies are becoming more cost efficient for both the patient and the fiduciary. More often than not, the patient is simply receiving a 2- or 3-day supply of immediate medication from either the doctor or pharmacists while the remainder of the prescribed dosage arrives directly to the patient’s residence. This delivery system has greatly reduced patient generated fraud, abuse, and black/brown market “doctor shopping.” Code D9630 is the code to use when delivering non-over-the-counter prescriptions and/or prescription strength medicaments directly to the patient. These could include antibiotics, analgesics, or prescription strength home use fluoride. This is not the code to bill when simply writing a prescription or giving away professional samples. Prescription writing is part of the evaluation and documented diagnosis. As concerns reimbursement, plans most often exclude benefits for take-home medicaments that can be used by anyone other than the patient. The classic example is again take-home fluoride. To eliminate confusion or misinterpretation, always identify in both the claim and treatment record the specific drug, concentration, dosage, and necessity.
|
Evidence-Based Dentistry, Step 2—Professional Experience and Judgment
Some believe that EBD takes away the dental professional’s ability to provide individualized patient care; that it is “cookbook” dentistry. Should a path exist that is mandated from diagnosis to treatment that everyone should or must follow, and is that a substitute for clinical judgment? This is not the case. Yet, how often, rather than using individualized clinical judgment, do we fall back on clinical traditions? These may or may not be based in science at all.
As a science-based profession, we must acknowledge that what we knew yesterday may not be the same as what we know today, and probably will not be the same as what we will know tomorrow. An example is the 6-month recall: It is so common that somehow we have led our patients to believe there is science behind it. Recall intervals should be individualized to each patient’s risk of developing new dental disease. There is little scientific evidence that the traditional 6-month recall for all patients results in improvements in oral health. So why do a majority of practitioners still recommend a 6-month recall? The answer is tradition, or the mistaken belief that because insurance policies cover procedures at certain intervals, those intervals are appropriate.
For decades, the ADA recommended that patients follow an oral home care regimen that included brushing twice daily with an ADA-accepted toothpaste and between the teeth with an ADA-accepted interdental cleaning device. In May 2007, the ADA began to advise that using an ADA-accepted mouthrinse is also useful. Yet the behavior and recommendations of many professionals has not changed; the ADA recommendations are rooted in EBD.
So, what is the bottom line? Professional judgment should be a part of EBD, not a stand-alone process. These recommendations should be combined with the third step of the professional EBD process: the patient’s presenting clinical conditions, as well as the patient’s personal preferences and values.
Evidene-Based Dentistry, Step 3—Patient Preferences/Values
The third key step in this nonlinear process is stated on the ADA EBD Web site as, “It is important to understand that EBD is an approach to practice, an approach to making clinical decisions, and is just one component used to arrive at the best treatment decision. EBD is about providing personalized dental care based on the most current scientific knowledge.”6 This is the key: personalized care. It has been somewhat traditional to attempt a one size fits all approach. It is easier to manage, schedule, and follow up, but unfortunately it doesn’t work well. Another approach taken by professionals is to recommend products based on their own personal tastes and experience. Although it is important for professionals to give a fair trial to products by using them personally, it is faulty thinking to believe that if something works for you, it will automatically be best for your patient.
People are individuals, with a wide variety of presenting conditions, allergies, and sensitivities. Needs and risk factors, as well as their own tastes, wants, and what fits in their lives should be considered and re-evaluated for individual effectiveness. Finding the right product for the right person sometimes takes several attempts. Often, professionals will fall into blaming the patient when something doesn’t work optimally; when, in fact, the product wasn’t the right choice for that person.
Answers for Ashley
In the brief description of Ashley’s case given above, do you have enough information to make a recommendation? You probably do not. Professionals may need to become “CSI detectives” to get the clues needed. At the same time, we need to stay mindful that Ashley is an adult. It could be easy to jump into a parental role and admonish her for her habits. If this happens, there is a likelihood that Ashley will not follow or even listen to the recommendations.
Prior to college, Ashley was a low risk. What clues to do we have that she has become a high risk? Her “cotton mouth” is something we can help her understand. It is possible that Ashley doesn’t correlate her choices to her oral condition. With energy drinks, professionals want to jump on the sucrose content; yet even with sugar-free energy drinks, other ingredients are potentially creating greater problems: the caffeine or other ingredients like taurine, guarana, ginseng, or ginkgo that add a similar effect mimicking caffeine. Caffeine causes dehydration and dry mouth, making those vod-bombs even more harmful. Antihistamines also exacerbate her dry mouth.
Ashley’s high-risk profile could be an easy fix; she does not report that she is using any mouthrinse. As a bioscience major, talking to her about biofilm and the 80-20 principle emphasizing routine disruption should be easy for her to understand. Ashley can be involved by using a chairside computer to view the mouthrinse science.
Research shows that when patients actively participate in their own care, the outcome is better.
References
- Reh FJ. Pareto’s principle—the 80-20 rule. management.about.com/cs/generalmanagement/a/Pareto081202.htm. Accessed December 20, 2011.
- Gurenlian JR. The role of dental plaque biofilm in oral health. J Dent Hyg. 2007;81(suppl):116.
- Costerton JW. New ammunition. Dimens Dent Hyg. 2007;5:14-16.
- Mealey BL, Rose LF. Diabetes mellitus and inflammatory periodontal diseases. Compend Contin Educ Dent. 2008;29:402-408, 410, 412-413.
- ADA Center for Evidence-Based Dentistry. About EBD. ebd.ada.org/About.aspx. Accessed December 20, 2011.
- Charles CH, Sharma NC, Galustians HJ, et al. Comparative efficacy of an antiseptic mouthrinse and an antiplaque/antigingivitis dentifrice. A six-month clinical trial. J Am Dent Assoc. 2001;132:670-675.
- Sharma N, Charles CH, Lynch MC, et al. Adjunctive benefit of an essential oil-containing mouthrinse in reducing plaque and gingivitis in patients who brush and floss regularly: a six-month study. J Am Dent Assoc. 2004;135:496-504.
- Wade WG, Addy M. In vitro activity of a chlorhexidine-containing mouthwash against subgingival bacteria. J Periodontol. 1989;60:521-525.
- Mankodi S, Bauroth K, Witt JJ, et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent. 2005;18(special issue):9A-14A.
- Roldán S, Winkel EG, Herrera D, et al. The effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc lactate on the microflora of oral halitosis patients: a dual-centre, double-blind placebo-controlled study. J Clin Periodontol. 2003;30:427-434.
- DePaola LG, Spolarich AE. Safety and efficacy of antimicrobial mouthrinses in clinical practice. J Dent Hyg. 2007;81(suppl):13-25.
- Frascella J, Gilbert RD, Fernandez P, et al. Efficacy of a chlorine dioxide-containing mouthrinse in oral malodor. Compend Contin Educ Dent. 2000;21:241-248.
- Shinada K, Ueno M, Konishi C, et al. Effects of a mouthwash with chlorine dioxide on oral malodor and salivary bacteria: a randomized placebo-controlled 7-day trial. Trials. 2010;11:14.
- Claydon N, Hunter L, Moran J, et al. A 6-month home-usage trial of 0.1% and 0.2% delmopinol mouthwashes. (I). Effects on plaque, gingivitis, supragingival calculus and tooth staining. J Clin Periodontol. 1996;23(3 pt 1):220-228.
- Lang NP, Hase JC, Grassi M, et al. Plaque formation and gingivitis after supervised mouthrinsing with 0.2% delmopinol hydrochloride, 0.2% chlorhexidine digluconate and placebo for 6 months. Oral Dis. 1998;4:105-113.
- Hase JC, Attström R, Edwardsson S, et al. 6-month use of 0.2% delmopinol hydrochloride in comparison with 0.2% chlorhexidine digluconate and placebo. (I). Effect on plaque formation and gingivitis. J Clin Periodontol. 1998;25:746-753.
- Addy M, Moran J, Newcombe RG. Meta-analyses of studies of 0.2% delmopinol mouth rinse as an adjunct to gingival health and plaque control measures. J Clin Periodontol. 2007;34:58-65.
- US Food and Drug Administration. Is the product a medical device? fda.gov/medicaldevices/deviceregulationandguidance/overview/classifyyourdevice/ucm051512.htm. Accessed December 20, 2011.
Ms. DiGangi and Ms. Bendit are national speakers who present on varied topics from electronic health records, risk assessment, instrumentation, and ergonomics to name a few. Together they created and present “Creating a Flight Plan Beyond the Routine,” a unique, first-of-its-kind continuing education experience very popular with many dental and dental hygiene groups. They can be reached at pdigangi.com or jzbeducate.com.
Disclosure: Ms.DiGangi and Ms. Bendit received an educational grant from Sunstar Americas toward the writing of this article.