INTRODUCTION
Antioxidants are prolific in our diets, being present in spices, herbs, fruits, vegetables, nuts, legumes, grains, and certain beverages. At a cellular level the body also produces natural antioxidants, and there are many supplements that either contain antioxidants or enhance the body’s ability to produce antioxidants. Studies have revealed a correlation between measurably low antioxidant levels with many inflammatory oral soft-tissue conditions and diseases.1,2 Recently, antioxidant products have been introduced in the marketplace that allow for immediate absorption into the oral cavity. Dental professionals should have a keen interest in understanding the role of antioxidants, the impact of oxidative stress, and some of the arsenals available to positively impact the oral environment.
UNDERSTANDING ANTIOXIDANTS, FEE RADICALS , AND OXIDATIVE STRESS
Antioxidants can be defined as molecules that prevent/delay oxidation and neutralize free radicals. Common examples of oxidation are seen when bananas or apples are cut open and exposed to the air and they begin to brown. Oxidation also happens inside the body at a cellular level during normal metabolic processes, but can be accelerated due to various exposures or when antioxidant levels are too low. Free radicals are molecules that are unstable as a result of having an unpaired electron and sources that create free radicals are common: exposure to ultraviolet light, radiation, air pollution, chemicals, tobacco, alcohol consumption, and in the oral environment include bleaching agents, dental cements, composite fillings, and metals used in dental procedures.3
Antioxidants counteract the process of oxidation by supplying a molecule to bond with the unpaired electrons of free radicals. They have the potential to interrupt or stop a chain reaction of free radical electron “stealing,” where one molecule steals an electron from another molecule, which in turn steals from another molecule and so on, creating a condition referred to as “oxidative stress.” Oxidative stress is the imbalance that results from too many free radicals in the body, or an inadequate amount of natural antioxidant defenses. Reactive oxygen species (ROS) and reactive nitrogen species are common types of oxidative stress seen in the body.
The imbalance of too much oxidative stress can lead to damage of the DNA, RNA, and protein enzymes. Chronic inflammatory processes also induce oxidative stress, thereby generating ROS.4 Particularly vulnerable molecules in the cell wall are unsaturated lipids. As early as 1987, data revealed that oxidative stress referred to as lipid peroxidation could initiate or ultimately promote the process of atherosclerotic lesion formation by directly damaging endothelial cells and increasing the susceptibility of platelets to aggregate.5 Endothelial cell injury by lipid peroxidation can also increase the uptake of low-density lipoprotein into the vessel wall.
Development and/or progression of many conditions have been linked to excessive oxidative stress and include kidney disease,6 nonalcoholic fatty liver disease,7 asthma,8 stroke,9 Parkinson’s disease,10 Alzheimer’s disease,11 cardiovascular disease,12 diabetes,13 cancer,14 and aging.15
Naturally occurring within the oral cavity are numerous antioxidant enzymes that help to maintain the homeostasis. Antioxidants can be measured directly from the saliva with specific assays or from the plasma as a measurement of total antioxidant capacity (TAC). Studies evaluating antioxidant levels have revealed a correlation between measurably low antioxidants or elevated oxidative stress with periodontal disease,16 lichen planus,17 oral cancer,18 recurring aphthous stomatitis,1 and dental caries.19 The right types of antioxidants and their bioabsorption can positively influence this unseen and unfelt interplay of cellular events in which oxidative stress could be either causative in nature or increases as a result of disease progression.
ANTIOXIDANT IMBALANCE AND PERIODONTAL DISEASE
Periodontal disease is one oral condition in which the release of proinflammatory mediators associated with pathogenic bacteria increases oxidative stress.
It is characteristic that patients with periodontal disease exhibit a reduced level of antioxidants. In fact, several studies have drawn conclusions about the relationship between antioxidant levels and oxidative stress:
- Periodontal disease is associated with reduced salivary antioxidant status and increased oxidative damage within the oral cavity.20
- Periodontitis, a common source of low-grade inflammation, is associated with a systemic oxidative stress state and ROS.21
- Oxidative stress is a critical factor in periodontal tissue damage that results from host microbial interactions as a direct result of excessive reactive oxygen species activity, antioxidant deficiency, or creation of proinflammatory markers.22
- Evidence for compromised plasma antioxidant capacity, independent of smoking, suggests an underlying environment of oxidative stress within periodontal patients.16
Interestingly, authors Chapple and Matthews16 concluded that their data, “provides opportunities to develop novel antioxidant therapies…which function not only as antioxidants in the traditional sense but also as powerful anti-inflammatory agents.”
ANTIOXIDANT IMBALANCE AND ORAL CANCER
Saliva, with its natural buffers, enzymes, and antioxidants, can form a powerful anticariogenic effect. However, there appears to be a consistent correlation between measurably low antioxidant levels in the saliva, and elevated oxidative stress and oral cancer. The following conclusions assist in appreciating of the role of antioxidants in the development, treatment, and even prevention of oral cancer:
- Oxidative DNA damage, a vital phenomenon for carcinogenesis of oral squamous cell carcinoma, occurs due to the interaction of oxidative stress and total antioxidant capacity.23
- Elevated oxidative stress markers correlated positively with recurrent squamous cell carcinoma of the oral cavity and a poor prognosis compared to patients staying in complete remission.24
- Oxidative stress levels were significantly higher in patients with oral squamous cell carcinoma but TAC was lower. This data indicates that patients with oral lichen planus and oral squamous cell carcinoma are more susceptible to an imbalance of antioxidant-oxidative stress status.17
- The oxidized proteins and DNA found in the saliva of oral cancer patients demonstrates a direct link among salivary free radicals, antioxidants, and oral squamous cell carcinoma.25
ANTIOXIDANTS FOR LOCAL ABSORPTION IN THE ORAL CAVITY
In addition to periodontal disease and oral cancer, there are many stressors to the oral cavity and numerous opportunities in which increased wound healing is desired. As arsenals to reduce oxidative stress in the oral cavity, and, to supply immediate absorption of important antioxidants, a manufacturer (PerioSciences) has introduced a novel array of antioxidant products. Currently, there are 2 types of antioxidant gels available: AO ProVantage (PerioSciences), and AO ProVantage BLAST (PerioSciences). AO ProVantage is a clear gel containing 2 antioxidants: phloretin, found in apples, and ferulic acid, found in the seeds and leaves of most plants. In addition, it contains menthol, thymol, essential oils, and xylitol. AO ProVantage BLAST contains the same formula but at a higher concentration of the antioxidants specifically targeting post nicotine exposure. Both AO ProVantage and AO ProVantage BLAST are applied with the fingertip directly to the tissue. AO ProRinse (PerioSciences) is a mouthrinse that contains ferulic acid, curcumin, and green tea catechins along with essential oils, menthol, aloe leaf juice, coconut water, and xylitol. This manufacturer formulates all products with pure antioxidants opposed to antioxidant extracts.
Patient experiences as well as ample literature reveal the significant impact tobacco use has on the oral environment, including increased risk for periodontal disease, vertical bone loss, and poor treatment outcomes. In vitro studies have also shown nicotine inhibits the attachment and growth of gingival and periodontal ligament fibroblasts.26 Based upon the hypothesis that nicotine impairs wound healing by increasing reactive oxygen species and inhibiting cell migration, research was conducted to investigate whether or not antioxidant combinations could counteract the nicotine effects that slowed cell migration.27 Both human gingival fibroblasts and human periodontal ligament fibroblasts that had been impregnated with nicotine were tested. Cell migration almost stopped in the presence of nicotine. Treatment with the antioxidant combination of ferulic acid and phloretin counteracted the effects of nicotine and stimulated cell migration.
Due to the fact that there are numerous inflammatory as well as acute and chronic conditions seen in the oral cavity, and ample exposures to oxidative stress, the potential benefit for local absorption of antioxidants is significant. Included in this article are case studies documenting integration of AO ProVantage products containing ferulic acid and phloretin into patient care for a variety of conditions (Figures 1a to 4d).
 |
 |
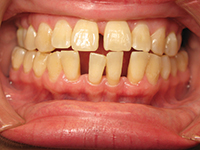
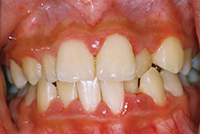
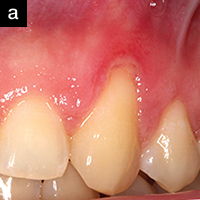
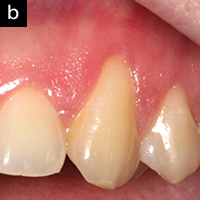
| Figure 1a. A 48-year-old at the completion of 6 sessions of scaling and root planing over a period of 180 days. Note unresolved inflammation. | Figure 1b. Three weeks after using AO ProVantage gel 2 times per day. Photos provided by Dr. Pat Allen, Dallas, Tex. |
 |
 |
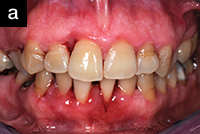
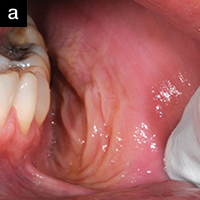
| Figure 2a. An 18-year-old referred for treatment of a localized gingival recession defect. The site exhibited severe erythema, heavy bleeding on probing, and suppuration. The patient was placed on AO ProVantge gel to control the inflammation prior to grafting the tissue defect. |
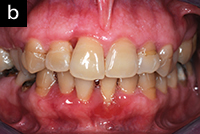
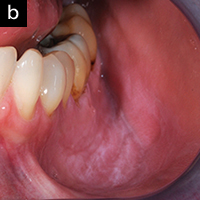
Figure 2b. After 2 weeks, with no treatment other than the AO ProVantage gel, the gingiva has significantly improved tone, reduced inflammation, and no suppuration or bleeding on probing. The patient is now ready for the gingival graft. Photos provided by Dr. Brian Young, Jacksonville, Fla. |
 |
 |
| Figure 3a. A 36-year-old patient who was a daily user of smokeless tobacco. He had developed a severe tissue reaction in the buccal vestibule. Regimen of 3 applications daily of AO ProVantage BLAST gel applied to the affected area. | Figure 3b. Six weeks following the patient complying with the regimen, although he continued to use the nicotine product. Significant resolution of the lesion in spite of continue dipping. Photos provided by Dr. Pat Allen, Dallas, Tex. |
 |
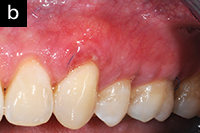 |
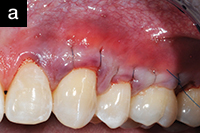
| Figure 4a. A 44-year-old patient who presented with bilateral recession defects involving 3 teeth in the maxillary left and right posterior areas. AlloDerm (BioHorizons) grafting was performed using the tunnel technique on 2 separate occasions. Platelet rich plasma was not used for this patient. Photo taken at time of suture. | Figure 4b. Three weeks postsurgery with use of chlorhexidine mouthrinse 2 times per day. |
 |
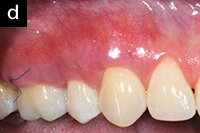 |
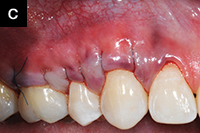
| Figure 4c. The same 44-year-old patient following second AlloDerm grafting procedure with the same technique used on opposite side. Photo taken at time of suture. | Figure 4d. Three weeks postsurgery with use of AO ProVantage gel 5 times per day instead of chlorhexidine mouthrinse. The patient reported preference for AO ProVantage gel due to its symptomatic relief upon use, pleasant taste, and lack of tooth staining. Photos provided by Dr. Pat Allen, Dallas, Tex. |
A SNAPSHOT OF SUPPLEMENTAL ANTIOXIDANTS
Antioxidant studies evaluating whether or not tested supplements decrease the risk of serious illness and disease or delay the aging process draw different conclusions depending upon how the study was designed, and the supplements tested. It does appear that diets rich in antioxidants provide some of the best sources for the body to absorb, but obtaining sufficient amounts of antioxidants from diet alone is unrealistic for the average consumer. A study published in the Journal of Clinical Periodontology in 2011 highlighted the value of adjunctive daily supplementation with fruit; vegetable and berry juice powder concentrates (for example, Juice Plus [NSA]) for periodontal patients who had measurably low antioxidant levels.28 This is not to suggest that this is the only antioxidant supplement of value to periodontal patients, but this was the first intervention study using randomized controlled trials to report improvements in pocket depth reductions with use of adjunctive juice powder concentrate supplements for periodontal patients. It also revealed lower bleeding upon probing and plaque scores at 8 months following supplementation.
One company that makes supplements using proprietary antioxidants also manufactures a novel technology that measures current antioxidant levels: Pharmanex. The BioPhotonic Scanner (Pharmanex) accurately measures the carotenoid antioxidant levels through the skin on the palm of the hand and is more reliable than measurement of serum carotenoid levels.29 The lower the number, the lower level of antioxidants present indicating higher oxidative stress systemically; and the higher the number, the greater presence of antioxidants, with lower oxidative stress systemically. Some medical and dental offices are incorporating use of the BioPhotonic Scanner with their patients to educate them on current antioxidant levels and oxidative stress, as well measure effectiveness of alterations to the diet coupled with supplementation several weeks later.
One novel supplement has emerged onto the marketplace that is distinctly different from other antioxidants in how it works. Protandim (LifeVantage) is made up of 5 antioxidants: milk thistle extract, bacopa extract, ashwagandha, tumeric extract and green tea extract. The synergistic effect of these antioxidants together activate the protein messenger NrF2 which in essence works at a cellular level to destroy free radicals, and increase the body’s own production of natural antioxidant enzymes such as superoxide dismutase, catalase, glutathione and others.30 Nrf2 is also capable of turning down the production of proinflammatory and profibrotic genes, which holds potential benefit for patients with many types of chronic inflammatory conditions in which elevated oxidative stress is detrimental, including those with periodontal diseases.
CLOSING COMMENTS
Dental patients who have an antioxidant/oxidant imbalance favoring the production of oxidative stress and low antioxidant capacity are at higher risks for the development and progression of many oral inflammatory conditions and diseases. The same antioxidant imbalance can predispose individuals to numerous chronic illnesses, cancer and accelerated aging processes.
New antioxidant products are emerging onto the marketplace almost daily. Novel therapies exist to increase antioxidant absorption directly into the oral tissue and improve clinical outcomes, and adjunctive dietary supplements have the potential to improve periodontal parameters. Supplements are available that will activate the body’s antioxidant production within the cells, and turn down the proinflammatory genes. There has never been a better time for dental professionals to increase their understanding of antioxidants and to integrate antioxidant products into their patient care regimen.
References
- Momen-Beitollahi J, Mansourian A, Momen-Heravi F, et al. Assessment of salivary and serum antioxidant status in patients with recurrent aphthous stomatitis. Med Oral Patol Cir Bucal. 2010;15:e557-561.
- Beevi SS, Rasheed AM, Geetha A. Evaluation of oxidative stress and nitric oxide levels in patients with oral cavity cancer. Jpn J Clin Oncol. 2004;34:379-385.
- San Miguel SM, Opperman LA, Allen EP, et al. Reactive oxygen species and antioxidant defense mechanisms in the oral cavity. Compendium. 2011 Jan/Feb;32:e10-e15. dentalaegis.com/cced/2011/02/reactive-oxygen-species-and-antioxidant-defense-mechnaisms-in-the-oral-cavity. Accessed July 20, 2012.
- Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499-510.
- Hennig B, Chow CK. Lipid peroxidation and endothelial cell injury: implications in atherosclerosis. Free Radic Biol Med. 1988;4:99-106.
- Crawford A, Fassett RG, Coombes JS, et al. Glutathione peroxidase, superoxide dismutase and catalase genotypes and activities and the progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:2806-2813.
- Morita M, Ishida N, Uchiyama K, et al. Fatty liver induced by free radicals and lipid peroxidation. Free Radic Res. 2012;46:758-765.
- Bauer M, Gräbsch C, Schlink U, et al. Genetic association between obstructive bronchitis and enzymes of oxidative stress. Metabolism. 2012 Jan 25. [Epub ahead of print]
- Nakajima H, Unoda KI, Ito T, et al. The relation of urinary 8-OHdG, a marker of oxidative stress to DNA, and clinical outcomes for ischemic stroke. Open Neurol J. 2012;6:51-57.
- Cloutier M, Wellstead P. Dynamic modelling of protein and oxidative metabolisms simulates the pathogenesis of Parkinson’s disease. IET Syst Biol. 2012;6:65-72.
- Puertas MC, Martínez-Martos JM, Cobo MP, et al. Plasma oxidative stress parameters in men and women with early stage Alzheimer type dementia. Exp Gerontol. 2012;47:625-630.
- Selvaraju V, Joshi M, Suresh S, et al. Diabetes, oxidative stress, molecular mechanism, and cardiovascular disease—an overview. Toxicol Mech Methods. 2012;22:330-335.
- Gilbert ER, Liu D. Epigenetics: The missing link to undertanding ß-cell dysfunction in the pathogenesis of type 2 diabetes. Epigenetics. 2012;7(8). [Epub ahead of print]
- Matés JM, Segura JA, Alonso FJ, et al. Oxidative stress in apoptosis and cancer: an update. Arch Toxicol. 2012 Jul 19. [Epub ahead of print]
- Wei YH, Lu CY, Wei CY, et al. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin J Physiol. 2001;44:1-11.
- Chapple ILC, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160-232.
- Agha-Hosseini F, Mirzaii-Dizgah I, Mikaili S, et al. Increased salivary lipid peroxidation in human subjects with oral lichen planus. Int J Dent Hyg. 2009;7:246-250.
- Barut O, Vural P, Sirin S, et al. The oxidant/antioxidant status and cell death mode in oral squamous cell carcinoma. Acta Odontol Scand. 2012;70:303-308.
- Southward K. The systemic theory of dental caries. Gen Dent. 2011;59:367-373.
- Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond). 2003;105:167-172.
- D’Aiuto F, Nibali L, Parkar M, et al. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89:1241-1246.
- Pendyala G, Thomas B, Kumari S. The challenge of antioxidants to free radicals in periodontitis. J Indian Soc Periodontol. 2008;12:79-83.
- Korde SD, Basak A, Chaudhary M, et al. Enhanced nitrosative and oxidative stress with decreased total antioxidant capacity in patients with oral precancer and oral squamous cell carcinoma. Oncology. 2011;80:382-389.
- Salzman R, Pácal L, Tomandl J, et al. Elevated malondialdehyde correlates with the extent of primary tumor and predicts poor prognosis of oropharyngeal cancer. Anticancer Res. 2009;29:4227-4231.
- Bahar G, Feinmesser R, Shpitzer T, et al. Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer. 2007;109:54-59.
- Fang Y, Svoboda KK. Nicotine inhibits human gingival fibroblast migration via modulation of Rac signalling pathways. J Clin Periodontol. 2005;32:1200-1207.
- San Miguel SM, Opperman LA, Allen EP, et al. Antioxidants counteract nicotine and promote migration via RacGTP in oral fibroblast cells. J Periodontol. 2010;81:1675-1690.
- Chapple ILC, Milward MR, Ling-Mountford N, et al. Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: a double-blind RCT. J Clin Periodontol. 2012;39:62-72.
- Zidichouski JA, Mastaloudis A, Poole SJ, Reading JC, Smidt CR. Clinical validation of a noninvasive, Raman spectroscopic method to assess carotenoid nutritional status in humans. J Am Coll Nutr. 2009;28:687-693.
- Hybertson BM, Gao B, Bose SK, et al. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234-246.
Ms. Davis is founder of Cutting Edge Concepts. She is an international speaker and practices dental hygiene in Dallas, Tex. She has served on numerous advisory boards in the profession, and is recognized by Dentistry Today as a Leader in Continuing Education. She is an accomplished author related to her passion of practicing on the cutting edge of the profession. She is an independent consultant to the Philips Corporation, to OraPharma Inc, and is affiliated with PerioSciences. She can be reached at karen@karendavis.com.
Disclosure: Ms. Davis has a financial interest in the companies PerioSciences and LifeVantage, mentioned in this article.