Dentists and laboratory technicians today require materials that offer outstanding aesthetics, high strength, and efficient productivity. Historically, dentistry also has always been faced with the challenge of finding ways to combine 2 incompatible materials in a synergistic way, whether that combination is metal with metal-ceramic or zirconia with zirconia layering ceramic. Simultaneously, dental practices and dental laboratories are increasingly seeking ways to realize the wonderful op-portunities presented by CAD/CAM and digital fabrication techniques that offer the benefits of consistency in the production of a restoration and expanded material options. To satisfy these requirements, lithium disilicate glass ceramic represents a unique material choice available to both doctors and laboratories. What’s more, lithium disilicate has the present and future potential to provide new options for improving patient care.
For the dentist, lithium disilicate is a highly aesthetic, high-strength material that can be conventionally cemented or adhesively bonded.1 In terms of dealing with dissimilar material combinations, lithium disilicate offers a unique solution with its ability to offer a full contour restoration fabricated from one high-strength ceramic, thereby eliminating the challenge of managing 2 dissimilar materials. Lithium disilicate is a material that can be used in all areas of the mouth, when specific considerations are taken into account. For laboratory ceramists, the versatility and performance of lithium disilicate enables them to optimize their productivity when fabricating restorations using this material, since either lost-wax pressing or CAD/CAM milling fabrication techniques can be used.
 |
 |
|
Figure 1. IPS e.max Press (Ivoclar Vivadent) lithium disilicate. |
Figure 2. IPS e.max CAD (Ivoclar Vivadent) lithium disilicate. |
 |
|
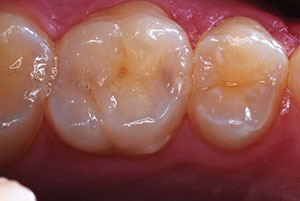
Figure 3. Posterior lithium disilicate crowns. Teeth Nos. 18, 19, and 20. |
The pressable lithium disilicate (IPS e.max Press [Ivoclar Vivadent])) is produced according to a unique bulk casting production process in order to create the ingots. This involves a continuous manufacturing process based on glass technology (melting, cooling, simultaneous nucleation of 2 different crystals, and growth of crystals) that is constantly optimized in order to prevent the formation of defects (eg, pores, pigments). The microstructure of the pressable lithium disilicate material consists of approximately 70% needle-like lithium disilicate crystals that are embedded in a glassy matrix. These crystals measure approximately 3 to 6 µm in length.
Polyvalent ions that are dissolved in the glass are utilized to provide the desired color to the lithium disilicate material. These color-controlling ions are homogeneously distributed in the single-phase material, thereby eliminating color-pigment imperfections in the microstructure.
Machineable lithium disilicate blocks are manufactured according to a similar process, but only an “intermediate” crystallization is achieved in order to ensure that the blocks can be milled efficiently in a crystalline intermediate phase (blue, translucent state). The intermediate crystallization process leads to the formation of lithium metasilicate crystals, which are responsible for the material’s processing properties, machineability, and good edge stability. It is after the milling procedure and the restorations are fired that they reach their final crystallized state and their high strength. The microstructure of intermediate crystallized IPS e.max CAD lithium disilicate consists of 40% platelet-shaped lithium metasilicate crystals embedded in a glassy phase (Figure 2). These crystals range in length from 0.2 to 1.0 µm. Postcrystallization microstructure of IPS e.max CAD lithium disilicate material consists of 70% fine-grain lithium disilicate crystals embedded in a glassy matrix.
Similar to the pressable lithium disilicate, the millable IPS e.max CAD blocks are colored using coloring ions. However, the coloring elements are in a different oxidation state during the intermediate phase than in the fully crystallized state. As a result, the lithium disilicate exhibits a blue color. The material achieves its desired tooth color and opacity when the lithium metasilicate is transformed into lithium disilicate during the postmilling firing process.
Table 1. Properties of IPS e.max Press.
|
Table 2. Properties of IPS e.max CAD.
|
PHYSICAL AND CLINICAL PROPERTIES OF LITHIUM DISILICATE
In vitro testing has included mechanical testing of strength using static load with a universal testing machine, subcritical eccentric loading using a chewing simulator (Willytec), and a long-time cyclic loading with a chewing simulator (eGo). The results of these tests demonstrate that:
- Regardless of the in vitro test performed, in comparison to various restorative dental material for crowns (eg, leucite glass ceramic, metal ceramic, zirconia), the lithium disilicate material demonstrates superior results.
- To ensure maximum success using the lithium disilicate material, it is important to consider the minimum thickness of the lithium disilicate.
- The strength of the ceramic material is about 80 to 100 MPa for metal ceramics, approximately 100 MPa for veneered zirconia, and 150 to 160 MPa for leucite glass ceramic. However, for the pressed lithium disilicate (IPS e.max Press LT and HT), the strength is in the range of 360 MPa to 400 MPa (Table 1).
The pressable lithium disilicate material is indicated for inlays, onlays, thin veneers, veneers, partial crowns, anterior and posterior crowns, 3-unit anterior bridges, 3-unit premolar bridges, telescope primary crowns, and implant restorations.3-5 In some cases, minimal tooth preparation is desired (eg, thin veneers), and lithium disilicate (IPS e.max [Ivoclar Vivadent]) enables laboratories to press restorations as thin as 0.3 mm while still ensuring a strength of 400 MPa. If sufficient space is available (eg, retrusion of a tooth), no preparation is required.
 |
 |
| Figure 4. Implant retained lithium disilicate restoration. |
Indications for the machinable lithium disilicate material are inlays, onlays, veneers, partial crowns, anterior and posterior crowns, telescope primary crowns, and implant restorations (Figures 3 and 4). For a posterior crown fabricated to full contour using CAD methods, lithium disilicate offers 360 MPa of strength through the entire restoration. As a result, restorations demonstrate a “monolithic” strength unlike any other metal or metal-free restoration.
Overall, these materials demonstrate specific advantages to dentists and patients, including higher edge strength versus traditional glass ceramic materials (ie, can be finished thinner) low viscosity of heated ingot enables pressing to very thin dimension (ie, enabling minimal prep or no prep veneers); and chameleon effect due to higher translucency (Table 2).
Conclusion
Lithium disilicate is emerging as a restorative material of choice for single unit indirect restorations. Lithium disilicate increasingly is being integrated into the North American and Western European dental practices. Not only is lithium disilicate strong, but it is very versatile and lifelike. It comes in many translucencies and can be layered to maximize aesthetics in select cases. The disilicate materials (IPS e.max Press and IPS e.max CAD) maximize these benefits for laboratories and dentists.
We have witnessed the many benefits of lithium disilicate and believe that it is now one of the best restorative materials available today for single unit indirect restorations. Lithium disilicate material (IPS e.max Press and IPS e.max CAD [Ivoclar Vivadent]) has been in clinical trials for the last 4 years with adhesive and self-adhesive/conventional cementation, and the results have been very positive. We continue to complete both in vivo and in vitro testing to enhance the material’s performance and maximize its use
clinically.
References
- Fabianelli A, Goracci C, Bertelli E, et al. A clinical trial of Empress II porcelain inlays luted to vital teeth with a dual-curing adhesive system and a self-curing resin cement. J Adhes Dent. 2006;8:427-431.
- Deany IL. Recent advances in ceramics for dentistry. Crit Rev Oral Biol Med. 1996;7:134-143.
- Sorensen JA, Cruz M, Mito WT, et al. A clinical investigation on three-unit fixed partial dentures fabricated with a lithium disilicate glass-ceramic. Pract Periodontics Aesthet Dent. 1999;11:95-106.
- Höland W, Schweiger M, Frank M, et al. A comparison of the microstructure and properties of the IPS Empress 2 and the IPS Empress glass-ceramics. J Biomed Mater Res. 2000;53:297-303.
- Kheradmandan S, Koutayas SO, Bernhard M, et al. Fracture strength of four different types of anterior 3-unit bridges after thermo-mechanical fatigue in the dual-axis chewing simulator. J Oral Rehabil. 2001;28:361-369.
Dr. Tysowsky is assistant clinical professor at the State University of New York at Buffalo, School of Dental Medicine, and is a Fellow of the American College of Dentistry. Dr. Tysowsky earned a DDS degree from the University of Minnesota, School of Dentistry, and a Masters of Public Health from Minnesota’s School of Public Health. He also completed a General Practice Residency at St. Francis Medical Center/University of Connecticut. He has published and lectured extensively throughout North America and Europe on the application of contemporary materials. He can be reached at george.tysowsky@ivoclarvivadent.us.
Disclosure: Dr. Tysowsky serves as vice president of technology for Ivoclar Vivadent. In this capacity, he is responsible for research and development activities for all North American operations.