A major concern for most dentists is how to treat periodontal disease successfully. Considering that it has been described by Williams1 as the No. 1 cause of tooth loss in the United States, and that it has been estimated that the disease affects more than 35 million Americans,2 management of periodontal disease has become an important issue in patient health.
WHAT IS PERIODONTAL DISEASE?
 |
|
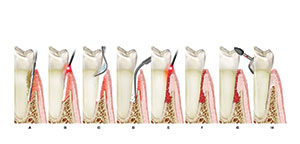
Figure 1. Host response to bacterial antigens produces periodontal breakdown. |
Periodontal disease is a common, chronic, persistent infection,3 with (according to the ADA) 3 out of 4 American adults developing a periodontal infection at some time in their lives.4 Harmful bacteria from the disease are able to spread rapidly throughout the periodontium, jeopardizing the gums, connective tissue, and alveolar bone, the foundation on which all dental work rests.5 It is critical for this disease to be diagnosed and aggressively treated using the latest technology and drugs.
Recently, we have determined what truly happens in the progression of this chronic disease. This now makes it easier for the dentist/hygienist to treat the disease with a predictable and successful result. Traditional periodontal treatment has been directed toward saving teeth and preventing bone loss. Until recently, we had presumed that the only way to control chronic periodontal disease was to eliminate the pathologic oral microbiota and inflammation with traditional methods of personal bacterial plaque control, scaling and root planing (SRP), and if necessary, periodontal surgery.6 Korman7 has explained the patient response to the microbial challenge in periodontitis as a response to bacterial antigens, resulting in periodontal breakdown (Figure 1).
MANAGING PERIODONTAL DISEASE
 |
| Figure 2. The Stat-Ck Record Sheet for scoring quadrant grades. |
Since we now have a better understanding of the causes of periodontal disease, we can predictably and successfully manage it. While it is recommended that complete recordings of periodontal probing readings be done, newer screening systems for the evaluation of disease with abbreviated probings and a scoring system may be used to allow a preliminary diagnosis that the patient can easily understand. Such a system is the Stat-Ck,8 which presents a treatment system based on recordings. The deepest pocket depth for each quadrant is selected, assigned a letter grade of A through F, and recorded on the Stat-Ck Periodontal Record sheet (Figure 2). Recommended treatment is listed for each grade category, based on the understanding of how the antimicrobial drugs work and our ability to combine this with traditional nonsurgical therapy. The grading system creates an increased awareness of both disease and health factors for the patient, and makes it easier for the patient to accept the necessary treatment to control the condition.
Specific bacteria (P gingivalis, T denticola, and T forsynthesis), known as the red complex, have been implicated in periodontal disease commonly found at the site of the infection. A direct association between these red complex bacteria, pocket depth, and bleeding on probing (BOP)—the most meaningful parameters in periodontal disease diagnosis—has been identified by Socransky.9
Due to the prevalence of periodontal infection and the persistent nature of the bacteria, and considering the potential serious nature of periodontal disease, all probings, charting, and treatment with SRP and adjunctive therapy should be done to prevent the spread of infection, loss of teeth, and need for surgery. Golub10 recognized that bacterial enzymes break down periodontal tissue, and he developed subantimicrobial doses of doxycycline (which exerts no antibiotic activity, although it is from the antibiotic family) to inhibit this enzyme activity in the gingival tissues and crevicular fluid. Caton, et al11 confirmed that the dose of 20 mg facilitated the reduction of periodontal disease progression in placebo-controlled studies.
There is an established need for mechanical instrumentation every 3 months to remove bacteria that produce destructive pathogens, since the bacterial organisms return to baseline levels within days due to the rapid multiplication rates of pocket bacteria.12 The return to baseline activity of these pathogens has redefined the parameters for treatment. Adjunctive treatment with the site-specific antibiotic Arestin (OraPharma) has been proven to affect the efficacy of SRP and help eliminate the bacteria, eg, P gingivalis, T denticola, and T forsynthesis, left behind by SRP. More than 60% of periodontal pockets responding to the site-specific anti-microbial Arestin had reductions of 2 mm or more.13
In a 2006 study by Goodsen, et al,14 Arestin in combination with SRP achieved a 49% reduction in red complex bacteria in 30 days, versus only 27% for SRP alone. This clearly establishes the immediate need for combined therapy if we are to successfully overcome the negative effects of red complex bacteria on the gingival tissue health.
The most recent study, published in 2008 and conducted by Novak, et al,15 found that the combined host modulation using low-dose doxycycline hyclate (20 mg, twice a day)—an adjunctive benefit to SRP16—and topical antimicrobial therapy in the management of moderate to severe periodontitis provided significantly greater clinical benefits than SRP alone. This again illustrates the immediate need for combined therapy to control the disease.
Luciak-Donsberger has concluded in the Journal of Evidence Based Dental Practice (2007) that the etiology of periodontal disease is clear, and there is strong scientific agreement that it is elicited by bacterial plaque or biofilm and modified by multiple risk factors. She has indicated that its severity depends on the host’s response to microbial infection, and that treatment during early stages is more effective than during later stages because periodontitis is a chronic disease, which over time leads to an increase in alveolar bone loss. A multitude of long-term studies have shown that periodontal diseases, in most cases, can either be avoided by primary prevention and arrested, or significantly slowed through secondary and tertiary preventive measures.17
It has often been thought that periodontal disease can be resolved by removal of the periodontally involved teeth. While this may reduce the periodontal risk, it has now become public knowledge that the removal of these teeth presents the consumer with potentially other serious risks. In a study published in Heart in September 2007,18 the influence of tooth loss on cardiovascular mortality was evaluated in an English population. There were 12,631 subjects at least 30 years of age studied at baseline. During up to 57 years of follow-up, researchers found that those with a large number of missing teeth in young adulthood, aged 9 or older, were one-third more likely to die of heart disease than their peers with fewer than 5 missing teeth, thus supporting the relationship between tooth loss and cardiovascular disease.
By knowing the cause of periodontal disease and administering both local and systemic antimicrobials as an adjunct to SRP, significant clinical benefits are provided by reducing probing depth and providing gains in clinical attachment to help control the disease successfully.
PERIODONTAL DISEASE AND CARDIOVASCULAR DISEASE
Current studies point to a role for periodontal disease in the development of cardiovascular and cerebrovascular diseases.19 While there is no direct data to show that treatment of periodontal disease will prevent cardiovascular events, Dr. Mark Reynolds (Chairman, Department of Periodontics, University of Maryland Dental School) has stated that a growing body of evidence now implicates chronic inflammation in the development of systemic conditions such cardiovascular disease. He feels that, given the well-established association between inflammatory periodontal disease and cardiac disease, dentists have an important responsibility to discuss with patients the potential impact of chronic infection on overall health in recommending treatment.20
A study in 2007 evaluated the effects of SRP and a subantimicrobial dose of doxycycline on oral and systemic biomarkers of disease in patients with both chronic periodontitis and coronary artery disease. It showed for the first time a preliminary response of statistically significant improvements in blood levels of total cholesterol, LDL cholesterol, triglycerides, and lipoproteins (all being reduced), and HDL cholesterol (good cholesterol…increased).21 This appears to be the first study to demonstrate the effects of this combined treatment on both periodontitis and systemic biomarkers of inflammation in cardiovascular disease patients. Further investigations are necessary to understand better the effect of this combined treatment on patients with both diseases.
PAINLESS TREATMENT
 |
 |
| Figure 3. Oraqix applicator tip placed in gingival sulcus. | Figure 4. Porter Nitrous Oxide Flowmeter. |
Finally, it is very important to provide the consumer patient with painless treatment. If consumers know that the treatment can be done painlessly, they will be more likely to accept the necessary care to control this disease. The most recent innovation in analgesia that makes it possible to do conservative treatment most of the time, without injectable anesthesia, is a thermogel (Figure 3) containing 2.5% lidocaine and 2.5% prilocaine, easily applied in the gingival sulcus with a cannula dispenser (Oraqix [DENTSPLY Pharmaceutical]). It has been found in 3 clinical studies to be effective in reducing pain during SRP.21 While it has been estimated that 15% of the US population declines dental treatment because they fear injections, another way to relax patients and reduce their perception of discomfort is with the use of nitrous oxide analgesia (Figure 4), eg, the Porter nitrous oxide unit (Porter Instrument Company). Yagiela22 has stated that it relieves anxiety and has specific actions that relieve discomfort. When used in combination with topical anesthetics, eg, Oraqix, the nitrous oxide, by its analgesic effect and because it alleviates anxiety, helps the anesthetic work better.
CONCLUSION
The patient can now expect to be treated successfully with this knowledge, complying as requested with preventive home measures. This now includes the daily use of a power toothbrush, irrigator, antimicrobial toothpaste, and mouth-rinse.19 By following the standard of care for nonsurgical periodontal treatment, patients can expect to maintain the periodontal health of their mouth successfully between dental visits and possibly reduce their risks for other serious medical conditions.
References
- Williams RC. Periodontal disease. New Engl J Med. 1990;322:373-382.
- Albander JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. J Periodontol. 1999;70:13-29.
- Page RC. Periodontal diseases: a new paradigm. J Dent Educ. 1998;62:812-821.
- American Dental Association. For the dental patient. Women and periodontal disease. J Am Dent Assoc. 2002;133:671.
- Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727-752.
- Gottehrer NR, Berglund SE. Antimicrobial host response therapy in periodontics: a modern way to manage disease. Dent Today. Sep 2006;25:84-87.
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33-53.
- Gottehrer NR, Shirdan TA. A new guide to nonsurgical management of periodontal disease. Dent Today. Sep 2002;21:54-59.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134-144.
- Golub LM, Wolff M, Roberts S, et al. Treating periodontal diseases by blocking tissue-destructive enzymes. J Am Dent Assoc. 1994;125:163-169.
- Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:521-532.
- Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12-55.
- Williams RC, Paquette DW, Offenbacher S, et al. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol. 2001;72:1535-1544.
- Goodsen JM, Gunsolley JC, Grossi SG, et al. Minocycline HCl microspheres reduce red-complex bacteria in periodontal disease therapy. J Periodontol. 2007;78:1568-1579.
- Novak JM, Dawson DR III, Magnusson I, et al. Combining host modulation and topical antimicrobial therapy in the management of moderate to severe periodontitis: a randomized multicenter trial. J Periodontol. 2008;79:33-41.
- Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Ann Periodontol. 2003;8:12-37.
- Luciak-Donsberger C, Piribauer F. Evidence-based rationale supports a national periodontal disease screening program. J Evid Based Dent Pract. 2007;7:51-59.
- Tu YK, Galobardes B, Smith GD, et al. Associations between tooth loss and mortality patterns in the Glasgow Alumni Cohort. Heart. 2007;93:1098-1103.
- Gapski R, Cobb CM. Chronic inflammatory periodontal disease. A risk factor for cardiovascular disease and ischemic stroke? Grand Rounds in Oral-Systemic Medicine. 2006;1:14-22.
- Gottehrer NR, Martin JL. The standard of care for nonsurgical periodontal treatment for reducing the dental risk for cardiac disease. Dent Today. Nov 2007;26:100-104.
- Donaldson D, Gelskey SC, Landry RG, et al. A placebo-controlled multi-centred evaluation of an anaesthetic gel (Oraqix) for periodontal therapy. J Clin Periodontol. 2003;30:171-175.
- Yagiela JA. Getting serious about laughing gas. Dimensions of Dental Hygiene. 2008;6:26-30.
Dr. Gottehrer has been in practice in suburban Philadelphia for more than 30 years, focusing his practice on cosmetics, implant dentistry, and periodontics. He is a graduate of the University of Maryland Dental School, received his postgraduate periodontal training at the University of Pennsylvania, and is a board-certified periodontologist. He teaches the Senior Elective Course in Periodontics at the University of Maryland Dental School. He has published and lectured nationally and internationally, and is currently the president of the Institute of Advanced Oral and Physical Health in Havertown, Pa. He can be reached at (610) 449-9500 or dr.neilg@verizon.net.