Asa has written that when dentists are going to make a change, they should not do so just for the sake of change, but rather to improve a given outcome.1 Further, he stated that the process of selecting a new dental material includes reading the literature (including the manufacturers’ instructions) and talking to colleagues. The manufacturers of dental materials are constantly introducing new versions of existing products, claiming that these new versions are improved. Furthermore, dental schools do not agree on the teaching of a protocol for the use of liners and bases.2 This implies that no single product or technique is the only correct choice. Consequently, the decision to use specific materials can be challenging for the clinician.
Table. Materials Used for Liners, Bases, and Cements.
*Temporary and permanent |
In an attempt to manage uncertainty regarding the use of liners and bases, it has been suggested that products currently in use be constantly reassessed.3 This paper will examine why clinicians use liners, bases, and cements, the types of materials that are currently available, and how they can be used. Examples of the types of products in each category are provided, but these examples are not the only products that are commercially available. Liners and bases will be discussed first, followed by a discussion of cements. As can be seen in the Table, certain materials can be used in more than one category.
WHY USE LINERS AND BASES?
The rationale for the use of liners and bases has changed over the years. Initially it was believed that prior to placement of a final restoration, a medicament was needed on the dentin surface to protect the tooth and pulp. Dental schools taught that this medicament was intended to protect the tooth from what was thought to be the toxic effects of the restoration, even though irritation from the restoration is mild and temporary. When postoperative pulpal inflammation did occur, the cause of this inflammation was bacteria and their byproducts within the dentin.4,5 Bacteria in the dentin can lead to severe inflammation and even necrosis of the pulpal tissues.6
A new model for the cause of postoperative sensitivity has emerged. According to Brannstrom,7 postoperative sensitivity is not caused by bacteria or their byproducts but is due to fluid that is found in the gaps at the tooth-restoration interface. It is the movement of this fluid that causes changes in osmotic pressures under the restoration, leading to postoperative sensitivity.7 More dentinal fluid is found in the tubules in deeper cavity preparations compared to that found in shallow ones, so there would be a greater osmotic effect with deeper restorations. Therefore, conservation of dentin would help combat postoperative sensitivity. In addition, an increase in the remaining dentin thickness will reduce the number of bacteria that reach the pulp. This was shown by Meryon, who demonstrated that increasing the remaining dentin thickness (RDT) by 0.5 mm would reduce the amount of bacteria reaching the pulp by 75%, and an increase in the RDT by 1.0 mm would decrease the bacteria by as much as 90%.8
Postoperative sensitivity may, in fact, be a result of a combination of these different concerns. Camps, et al reported that the factors influencing pulpal response are the following (in decreasing order): presence of bacteria, the remaining thickness of dentin, and postoperative delay (the time between cavity restoration and pulpal inflammation). The presence of bacteria on the cavity walls is the main factor influencing the pulpal reaction under restorative materials, not the restorative material itself.9 In contrast, Murray and coworkers observed differences in the ability of restorative materials to prevent bacterial leakage and pulpal inflammation. In order from most effective to least effective were resin-modified glass ionomers, bonded amalgam, zinc oxide eugenol, and composite resin.10
Teeth with a thin RDT can also experience postoperative discomfort due to thermal shock. This is especially true for metal restorations, since temperature changes transmitted through the restoration can cause fluid movement in the dentin.11
Microleakage is defined as the flow of oral fluid and bacteria into microscopic gaps between the prepared tooth surface and the restorative material.12 This movement of extracoronal fluids and associated debris into the dentin can cause or be associated with secondary caries, pulpal inflammation, pulpal necrosis, and periodontal disease.13 Currently, there are a number of methods for preparing teeth for restorations, including use of a bur, air abrasives, lasers, and hand instruments. The method by which tooth structure is removed does not affect microleakage.14
For patient comfort, the reduction/elimination of microleakage and the elimination of any gaps between tooth structure and restorative material must be achieved. Liners, bases, and cements help achieve this end.
TYPES OF LINERS AND BASES
Desirable properties for liners and bases include the absence of adverse effects on the pulp, high compressive and tensile strengths, and radiopacity.15 Certain ideal properties of temporary cements would also be desirable in liners and bases. Ideal features of temporary cements include controlled dispensing, compact kit (resulting in minimal waste), easy mixing and easy cleanup, and rapid setting.16 The ideal liner or base would have properties from both lists. When using any such material, proper isolation is essential to reduce bacterial contamination of the cavity preparation. Use of the rubber dam is the ideal method for achieving isolation.
Numerous products can function as a liner, base, or cement. These can include varnish and calcium hydroxide-based, zinc oxide eugenol/noneugenol-based, zinc phosphate-based (zinc oxyphosphate), zinc polycarboxylate-based, glass iono-mer-based, or resin-based products (Table).
LINERS
A liner is defined as a material that is applied in a thin layer, usually 0.5 mm thick, to seal the dentin on the floor and walls of a cavity from the influx of bacteria or irritants from restorative materials and procedures.17,18 Additionally, a liner (other than a varnish) may provide some therapeutic benefits.19 Varnish, calcium hydroxide, zinc phosphate, glass ionomer, and resin can be used as a liner.
BASES
Bases are applied in thick layers to provide the pulp with thermal protection. These materials must be strong enough to support a restorative material during placement and function.18 They encourage the recovery of the injured pulp. Essentially, bases serve as a replacement or substitute for dentin that has been destroyed by caries or removed during cavity preparation.20 Bases can be shaped and contoured to specific forms.17 Craig and Powers19 subdivided bases into low strength and high strength categories, with the low-strength bases referred to as liners. Varnishes and calcium hydroxide materials are not suitable for use as a base.
VARNISH
Dentists who recently completed training will usually use the restorative techniques they were taught in dental school. The number of schools teaching the use of varnish has decreased over time.3 The purpose of placing a varnish (eg, Copalite, Cooley & Cooley; Copaliner, Bosworth) is to seal the dentinal tubules, which will reduce the effects of microleakage. This is accomplished as the solvent portion of the liquid (which is usually organic in nature and includes acetone, ether, or chloroform) evaporates after placement. This leaves the solute, which is a natural gum (resin or copal) to seal the dentin.20
Varnishes are placed in the cavity preparation prior to placement of an amalgam restoration. Over time, the varnish will be dissolved by oral fluids and replaced by the corrosive byproducts of the amalgam.21 In addition to sealing the dentin, a varnish may prevent the metal ions from migrating from the amalgam to the tooth; this is responsible for the darkening of the tooth adjacent to an amalgam.18 Due to the fact that varnishes seal the dentinal tubules, they are contraindicated when the final restoration involves a resin-based material.
Several varnishes contain fluoride. They were first fabricated about 35 years ago to provide clinicians with an agent that extended the time that fluoride was in contact with the enamel.22 When combined with other fluoride treatments, the use of fluoride varnishes has been associated with a decline in the rate of caries.23 Further, one fluoride varnish has been shown to reduce microleakage when used as a luting agent for provisional crowns. This agent also increased the retention of a zinc oxide eugenol-based temporary cement.24
The FDA has approved the use of fluoride varnishes (eg, Duraphat, Colgate Oral Pharmaceuticals; Durashield, Sultan Dental Products) as cavity liners and to treat hypersensitive teeth. The FDA has not approved the use of varnishes as an anticaries agent.
The means by which fluoride varnishes reduce caries is similar to that of a mouthwash containing fluoride. Calcium fluoride
is deposited on the tooth surface and then converted to fluoroapatite via a remineralization reaction. The extended time of surface contact that fluoride varnishes have as compared to fluoride mouthwashes is advantageous, especially to those at risk for root caries.19 The disadvantage of these materials is the bittersweet taste and short-term (usually 24 hours) discoloration of the tooth.
It has been observed that some fluoride varnishes do not appear uniform (presence of dark streaks) when dispensed. Shen and Autio-Gold have shown that fluoride content can vary between doses when dispensed from the same tube.25 In addition, some contraindications to the use of varnishes have been identified. For example, varnishes can decrease the surface hardness of glass ionomers.26
Lastly, varnishes do not have multiple uses; they can only be used as a liner and not as a base or cement.
Calcium Hydroxide
Calcium hydroxide (CaOH) is a material whose popularity is declining. This is ironic, since CaOH has several advantageous properties. One of these is the antibacterial property of the catalyst portion of the material.27 The acidic nature of bacterial byproducts are neutralized by CaOH, which has a basic pH (pH of about 11). This pH means that the material acts as an irritant, which leads to the formation of reparative dentin. Furthermore, CaOH has the capacity to extract growth factors from the dentin matrix, causing the formation of a dentin bridge.28
Calcium hydroxide (eg, Dycal, DENTSPLY/Caulk; Pre-line, Schein) products are avail-able in both light-cured and self-cured versions, and their compressive strength continues to increase over a 24-hour period.19 Despite this increase and the fact that CaOH is some-times referred to as a base, these products should only be used as liners. CaOH is highly soluble in water, and marginal leakage will wash out the material. This may explain why it has been concluded that CaOH will not provide a long-term seal.29 Should the material dissolve, this would leave a restoration that is, to some degree, unsupported and thus prone to fracture.
Zinc Oxyphosphate
This material has been available for many years and is usually considered both a base and a cement. Zinc oxyphosphate (ZOP) makes an ideal base, since it can be shaped and contoured within several minutes of being placed. Amalgam can be condensed within several minutes after placement of ZOP.
ZOP products (eg, Fleck’s Cement, Mizzy) are available as a powder-liquid combination, where the powder contains zinc oxide (90%) and magnesium oxide (10%). The liquid contains orthophosphoric acid. The liquid gives ZOP an initial pH of about 3.5, which rises to 6.9 after about a week.20 It was thought that postoperative sensitivity when a ZOP product was used was due to the acidic nature of the product, which led to the use of a varnish on exposed dentin prior to the use of ZOP.3
Since heat is generated upon mixing the powder and liquid, it is advised that the spatulation be performed on a cold glass slab. Further, this will result in an increase in working time and will allow more powder to be incorporated into the mixture, which will enhance the physical properties. The glass slab needs to be completely dry prior to mixing, because any moisture on the mixing surface will cause the cement to set faster.
ADA Specification No. 96 applies to zinc phosphate and other water-based cements. (Water-based cements are those materials that have water as at least one component of the liquid portion of the product.) This specification states that ZOP packages must contain 20% more liquid than what is needed to mix with the corresponding powder. This will account for the liquid that will evaporate when the package’s cover is removed. Loss of water from the liquid component will cause the mixture to be more acidic, which will make it less biocompatible. Using ZOP as a base will require the mixture to be more viscous, resulting in a putty-like material that can easily be applied to the floor of a cavity preparation.19 When mixed in this form, there is less free acid available to irritate the tooth and pulp.
One ZOP product (Doc’s Best Copper Cement, Cooley & Cooley) has been proposed for use as a liner and base because, as the manufacturer claims, the copper component is antibacterial. Additionally, since one version of the product is not tooth-colored (brown), it is ideal for use as a base. In the event the tooth needs to be re-prepared, the brown color differentiates ZOP from the floor of the cavity preparation.
Zinc Oxide Eugenol
Zinc oxide eugenol (ZOE) provides a superior marginal seal. Mixtures with a lower powder-to-liquid ratio provide a better marginal seal than those with a higher ratio.30 This characteristic, along with its neutral pH, provides a sedative effect. This effect can also be attributed to antibacterial properties of these materials.31 ZOE is also a mild irritant to the pulp. This irritation causes a chronic pulpal inflammatory response, resulting in the formation of reparative dentin. This is especially true in deep cavity preparations where the water in dentinal tubules causes the eugenol to leach out of the ZOE.32,33 Eugenol has been shown to interfere with cellular respiration, which may result in pulpal necrosis.34 Therefore, ZOE (eg, IRM, DENTSPLY/Caulk) materials should not be used as direct pulp-capping agents.
Zinc oxide eugenol materials are available in 4 different varieties, relating to the manner in which they are to be used. Type I is considered a temporary or short-term cement; type II is a permanent or final cement; type III is for temporary restorations; and type IV is a cavity-lining material.35 When a ZOE material is to be used as a base, clinicians should allow approximately 24 hours after the placement of this material before the placement of a final restoration, since the ZOE cannot initially withstand the forces of condensation.
Zinc Polycarboxylate
Introduced in the 1970s, zinc polycarboxylate (ZPC), also known as zinc polyacrylate, is similar to ZOP. The main difference between the 2 is the liquid component. With ZPC, the liquid is polyacrylic acid. Although it may be difficult to mix and handle, ZPC is adherent to tooth structure. This is the result of the interaction between the carboxylic acid and the calcium ions in the dentin. The size of a polyacrylate acid molecule is thought to be too large to penetrate the dentinal tubules and thus reach the pulp, and this characteristic is believed to give ZPC biocompatibility.17
ZPC (eg, HyBond Polycarboxylate, Shofu; Durelon, 3M ESPE) has a very short set time, making it a user-friendly base. As with ZOP, ZPC should be mixed on a cold glass slab to increase the working time. When used as a base under amalgam, ZPC can be placed below any thin enamel so that the metallic color of the amalgam does not show through.
Glass Ionomers
This category of material can be used as a liner or a base and has been available for more than 30 years. Glass ionomer (GI) materials are a powder-liquid product where the powder is a silicate glass that contains calcium, aluminum, and fluoride.18 The liquid is polyacrylic acid.
Glass ionomer products are available in hand-mixed formulations (eg, Vitrebond, 3M ESPE), encapsulated/ cartridge formulations (eg, Ketac-bond Aplicaps, 3M ESPE; Fuji Lining LC, GC America), and self-cured or light-cured varieties. Conventional GI has a lower sealing ability than the light-cured (resin-modified) materials.36
Glass ionomers offer 2 advantages. The first advantage is the ability to bond to tooth structure. This is an ionic bond between the carboxyl groups of the GI and the calcium ions in the tooth.37 With respect to fluoride release, when a glass ionomer is placed at the margin (as in the sandwich technique) or as a cement, it has been found that there is a reduction in recurrent caries due to the release of fluoride.38 The fluoride in GI allows for the remineralization of the adjacent tooth structure.39 When a GI is used as a liner, there is a reduction in the problems associated with microleakage. This has been attributed to its antimicrobial properties.40 Additionally, as opposed to the aforementioned materials, when an adhesive restoration is placed over GI it can be bonded to the GI. Another advantage of GI is that the use of a resin-modified glass ionomer as a liner significantly reduces volumetric polymerization contraction for light-cured resin restorations.41
Glass ionomers are usually not promoted as a base but rather for use as a core build-up product (eg, Fuji IILC Core Material, GC America; Vitremer, 3M ESPE). However, they can be used as a base since they meet the characteristics of a base, as noted previously. There is a benefit to using GI as a base. One study demonstrated that the partial removal of caries and placement of GI or composite material over a CaOH liner, followed several months later by removal of the filling material, resulted in a hard and dry dentinal surface.42 This technique appeared to result in a reduction in the number of microorganisms found in the cavity preparation.
Glass ionomers do have disadvantages. These materials should not be used as direct pulp-capping agents, since they will cause a persistent inflammatory pulpal response. This is thought to be due to a lack of formation of dentinal bridges.43 This is contrary to another report in which it was stated that pulp tissue reactions are no different than those seen with ZOE.19
As with ZOP and ZPC materials, glass ionomers can be mixed on a cold glass slab in order to slow the setting time. However, this can have an adverse effect on the strength of the final mix.19 Clinicians need to remember that when mixing GI as a liner, the powder-to-liquid ratio will be reduced compared to when this material is mixed as a cement. This will result in a lower pH for a longer period of time, possibly leading to short-term postoperative sensitivity. A stronger bond of GI to dentin can be achieved if the cavity preparation is first cleansed. It has also been shown that conditioners (such as polyacrylic acid or phosphoric acid) significantly improve the bond to enamel for both conventional and resin-reinforced glass iono-mers.44 This may be useful with respect to teeth that suffer from fluorosis, as it has been demonstrated that fluorosis reduces the shear bond strength of GI to dentin.45
Resin
The newest category of liner and base, and the one that is quickly becoming popular, is resin. Resins generally have low solubility, are available in numerous shades and different viscosities, and are high in compressive and tensile strength. This paper will subdivide this category into primers/bonding agents (unfilled resins) and filled resins.
With respect to resins as cavity liners, it should be remembered that it is the dentin bonding agent that comes in contact with the dentin, not the filled resin. This is the layer that the clinician needs to evaluate for effectiveness. Over time, clinical techniques have changed. Initially, adhesive dentistry (bonding) was performed without any acid etching of the dentin. Currently, etching of the dentin is standard procedure, with the etchant material contained within the dentin bonding agent. These products became the sixth generation of bonding agents. The sixth generation versions are of 2 types. Type 1 (eg, Clearfil SE, Kuraray) requires that the components (self-etching primer and ad-hesive) be applied separately. With Type 2 products (Adper Prompt L-Pop, 3M ESPE), the components are mixed together prior to application. These materials form what is termed a hybrid zone. This hybrid zone serves both to seal the cavity surface and to provide retention of the restoration. Generally, these sixth generation products have a lower pH, making them incompatible with self-cured resins.
These dentin bonding agents are technique sensitive. This is due to the number of steps involved. One question is wheth-er or not the dentin should be kept moist, and if so, how moist? Clinicians should remember that as the cavity preparation approaches the pulp, the dentin has a higher fluid content. This can reduce the bond strength of the material.46,47 Since different areas of a cavity preparation are not uniformly wet due to hy-draulic forces, Pashley and Tay recommend that it is better to overdry the tooth, especially in deep preparations.48 Therefore, it is important for the clinician to understand and follow strictly the manufacturer’s instructions. Peutzfeldt and Vigild reported that the degree to which dentists complied with the instructions for use was influenced by the degree that dentists were satisfied with the instructions the manufacturer provided. 49
Recently, a seventh generation of bonding agent has been introduced. These add components to previous generation products that make them a single-component system (eg, I-Bond, Heraeus Kulzer; G-Bond, GC America). In some cases these products are antibacterial50 and reduce the number of steps involved for the clinician. Generally, these materials have a very low film thickness. This is important, because if proper technique is used, there will be no pooling of the material, as can happen with unfilled resins. The result would be that postoperative radiographs would not exhibit a radiolucent line under the final restoration that might be confused with recurrent decay.
There are indications that adhesives placed under amal-gam restorations reduce microleakage.51,52 This technique has an additional advantage. The placement of an adhesive into a cavity preparation along with amalgam (amalgam bonding) will partly restore the strength and rigidity lost by the cavity preparation. This may also lead to a reduction in cuspal flexure and the incidence of tooth fracture due to fatigue.53 Dentin-bonding agents (DBA) are not recommended for use as a direct pulp-capping agent since they, like glass ionomers, do not promote the formation of dentin bridges.54 In addition, DBA can exert harmful effects on the pulp.55
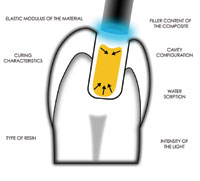
The use of composite resin to restore teeth has increased over the past several years. It is well-known that these resins are affected by polymerization shrinkage, which can lead to gap formation at the resin-tooth interface. To overcome the effects of this shrinkage, manufacturers have developed a diluted resin known as a flowable composite. The concepts behind the use of these products are their reduced elasticity and better flow. These 2 factors allow for an increase in the adaptation of the material to the cavity preparation and the forming of a stress-absorbing layer.56 This occurs even after one considers that flowable resins have a reduced filler content, which allows for an increase in polymerization shrinkage. The net result is a decrease in the gap and reduction in microleakage, secondary caries, and pulpal inflammation. Ultimately, this will lead to a more durable restoration.57
As noted earlier, considering that bond strength weakens as the RDT decreases, it has been demonstrated that the weakest bond is at the junction of the flowable resin and the tooth. Polymerization of the resin will allow gaps to form.58 Christensen has noted that many dentists use flowable composites (eg, Tetric Flow, Ivoclar Vivadent; Virtuso Flowable, DenMat) as a cavity liner even though these products are not intended for that purpose. He recommends using a fluoride-releasing material instead.59 According to the manufacturers, many flowable resins are said to release fluoride. However, it has been reported that any fluoride release is low and that the rate steadily and significantly decreases during the first 3 weeks.60
Over the past several years, new curing lights have been introduced. It has been shown that some of these lights have limited ability to polymerize some resin products. Some products, it appears, require more cure time than the manufacturers suggest. Therefore, those practices using narrow spectrum lights, including most LED lights, need to confirm that the light and the resin they are using are compatible.61
CEMENTS
The process of selecting appropriate cement/luting materials can be complicated by the fact that certain product descriptors can be misleading. For example, some products have the words “lining cement” in their name even though the product is not designed to be used as a cement. Others are called cements (specifically when referring to glass ionomers) even when the product is intended to be used as a restorative material, not a luting agent. Therefore, clinicians must be careful when choosing a new product and make their selection based on the product’s intended use, not its name.
Cements serve to retain restorations or appliances in a fixed position in the mouth.19 A dental cement, also known as a luting agent, acts as a barrier against microleakage by sealing the interface between the tooth and restoration and holding them together via some form of attachment. This attachment is mechanical, chemical, or a combination of both.37
Dental cements can be considered as temporary, short term, or weak, or they can be referred to as permanent, long term, final, or strong. Dental cements are only indicated when dealing with fixed prostheses. Varnishes cannot be used as a cement.
Following is a discussion of the types of cement/luting materials.
Calcium Hydroxide
Calcium hydroxide products (eg, Dycal, DENTSPLY Caulk; Life, Kerr) are available in both light-cured and self-cured varieties. Although not usually considered to be a cement, these products are an ideal temporary cement. This is because the material is easily applied, easily cleansed from the margins of temporary or permanent crowns, allows for easy removal of temporary crowns, and does not contain eugenol (which would interfere with resin-based final cements).
The light-cured varieties of CaOH are not recommended as temporary cements for castings or temporary crowns, as the curing light will not reach the material.
Zinc Oxide Eugenol/
Noneugenol Zinc Oxide
Of the 4 types of ZOE that exist, only 2 are intended for cementation. Type I is considered a temporary or short-term cement (eg, Tempbond, Kerr), and Type II is a permanent or final cement (eg, Fynal, DENTSPLY/Caulk).
There are reports that ZOE materials have an ad-verse effect on resin-based products.62,63 Other data do not support this conclusion.64 Re-placing the eugenol with carboxylic acid results in the noneugenol zinc oxide (non-ZOE) formula. Unlike ZOE, these products do not have a sedative effect on the pulp. Compared to ZOE products, non-ZOE products are slower to set and do not soften provisional acrylic crowns.19 Never-theless, Christensen states that the need for noneugenol zinc oxide materials (eg, NoMix, Centrix; Prevision, Heraeus Kulzer) prior to final cementation with resin cement is not a documented necessity.65
At present, ZOE is not popular as a final cement, but is popular as a temporary cement. The same is not true for non-ZOE materials, as they can be used for both temporary and final cementation.
Zinc Phosphate
Retention using zinc phosphate is due mainly to a physical interaction as opposed to a chemical reaction. Therefore, any coating placed on the tooth surface for pulpal protection will reduce retention. A study by Johnson and co-workers66 demonstrated that the use of a resin sealer with ZOP decreased casting retentive stress by 42%. The postoperative discomfort associated with the use of ZOP is likely due to the poor sealing capability of this material.
ZOP (eg, Fleck’s cement, Mizzy) should be dispensed when the material is ready to be used. If the material is dispensed and sits open prior to use, water will evaporate from the liquid component (the same is true for all water-based cements). The result will be a material that is too viscous, thus making it more difficult to seat castings. Loss of water can also lengthen the setting time of the product.
It has been suggested that a varnish or other nonpermeable coating should be placed at the margins of castings to allow the zinc phosphate to mature and thereby develop an increased resistance to dissolution by oral fluids. This should be done after the excess cement has been removed from the margins.20
With respect to the ability of ZOP to resist microleakage, the research is not conclusive. Zinc phosphate has been shown to allow the most microleakage when compared to other types of luting agents, but it has also been shown to be effective at resisting microleakage.67,68
As previously mentioned, ZOP has a long history of use in dentistry. In fact, more than 100 years ago zinc phosphate products containing 7% cupric oxide were introduced. The rationale for adding the cupric oxide was that the copper component made the material germicidal. In its original formulation, the copper gave the material a reddish-brown color, which would make it unsuitable for use with modern, nonmetallic restorations. Copper cement is now available in 2 colors, original and white (Doc’s Best Copper Cement, Cooley & Cooley).
Zinc Polycarboxylate
Zinc polycarboxylate materials have been available for approximately 30 years. The liquid contains polyacrylic acid (which makes it viscous), and when added to the powder, it can produce a mixture that has a higher film thickness. Therefore, if not properly mixed, the viscosity may interfere with the complete seating of castings.69 Polycar-boxylates are also known to be pseudoplastic, meaning that the material, when mixed to the correct viscous consistency, will still flow back under its own weight.19 Some ZPC products have stannous fluoride added in an attempt to reduce the film thickness.
ZPCs (eg, Hy-bond Poly-carboxylate, Shofu; Livcarbo, GC America) initially have a pH of about 1.7 (due to the acid). The pH quickly rises to neutral when mixed with the powder. This type of material has a rubbery consistency as it sets, and therefore any excess should be removed only after the cement has completely set. Removal prior to complete set could result in a tearing of the material from under the margin. This would result in gap formation at the tooth-res-toration interface.
When using ZPC, the clinician should be aware that the cement-casting interface is where failure may occur (ZPC chemically adheres to the tooth). This is in contrast to ZOP, where the cement-tooth interface is where failure can occur.17
Li and White have shown that compared to other luting agents, the modulus of elasticity of polycarboxylates (and glass ionomers) increases over time.70
Glass Ionomers
GI materials can be used as cementing agents in addition to being used as a liner or base. Some of these products have a hydrophilic monomer component. These materials are then referred to as resin-reinforced or resin-modified glass ionomers.12 Resin-modified glass ionomer cement (eg, FujiCem, GC America) has the added advantage of fluoride release (especially important for patients with increased caries risk), moderate strength, and ease of use.71 GI can also be used as an orthodontic cement. The advantage in this situation is that due to fluor-ide release, the material will minimize decalcification associated with orthodontic appliances. The fluoride in GI that is released can be replaced. This can be accomplished with toothpastes, topical gels, and mouth-rinses. Fluoride gels are the most effective means of achiev-ing fluoride replacement.72,73
One must be careful when using GI as a cement, specifically when the margins of fixed prostheses are susceptible to contact with moisture during the period of set. GI materials are known to be sensitive to moisture and should be protected from water and saliva contamination for at least 15 minutes after placement.74 It has also been suggested that a resin adhesive be used as a surface protection agent to reduce marginal microleakage during the initial setting period.75
It has been reported that glass ionomer-based materials (which are hydrophilic) expand when they come in contact with water. Therefore, the marginal seal of castings luted with these materials may be improved due to this hygroscopic expansion and may also exhibit greater bond strength.76 However, this expansion is the most likely cause of fracture of all-ceramic restorations luted with glass ionomer cement and may explain why some manufacturers are developing GI cements with lower expansion properties.77 Additionally, GI cements exhibit significantly less solubility than either zinc phosphate or zinc polycarboxylate cement.78
As mentioned earlier, glass ionomer products are available in hand-mixed (eg, CX-Plus Glasionomer Cement, Shofu), encapsulated, (eg, Vivaglass, Ivoclar Vivadent), and cartridge (eg, RelyX Luting Plus, 3M ESPE) versions. The hand-mixed formulations have been shown to have a significantly greater compressive strength.79
Resin
Since resins are available as self-cured, light-cured, or dual-cured materials, they can be used as a cement or luting agent both on a temporary (short-term) and permanent (final) basis. Additionally, since these materials are tooth-colored and are available in numerous shades, they are well-suited for luting aesthetic restorations. Because of their ability to bond with tooth structure, they can be used in situations involving nonideal prep-arations. In fact, when using a temporary resin cement (eg, TNE Noneugenol Temporary Cement, Temrex), it may occasionally be difficult to remove the temporary restoration.
The chemical-cured products (eg, Multilink Universal Luting Cement, Ivoclar Viva-dent) have a short working time, since the material begins to set upon mixing. However, they can be used under a metal, resin, or ceramic restoration regardless of its thickness. The light-cured products (eg, IntegraCem, Premier) are photoinitiated and have a greater working time. This reduces the darkening effect that can be seen with the self-cured materials. These products need to be protected from ambient light between the time they are dispensed and when they are used. The dual-cured products (eg, Permacem, Zenith/
DMG) are ideal when a thick or opaque restoration is to be luted (curing lights are unable to penetrate beyond 4.0 mm). When using these cements, the clinician is able to light cure at the margin and thereby hold the restoration in place, while the remainder of the cement that the curing light cannot reach is setting.80
With respect to microleakage, a comparison has been performed between acid-base cements and a resin cement. The resin cement exhibited sig-nificantly less microleakage than the other luting agents.81
There are some clinicians who believe that adhesive dentistry cannot be performed on a tooth that has been treated with a ZOE-based material. However, there are reports that refute this.82,83 Further, when cementing a post, resin cements are initially the most retentive, although over time ZOP is stronger. This has been attributed to the remaining unset root canal sealer or the eugenol in the sealer that remains in the canal.84 These authors go on to explain that if the post space is made at the time of obturation, then it probably does not matter which type of cement is used. However, if the post space is made a month or more later, one should avoid using a resin cement for post cementation because the eugenol has permeated into the dentin, and this will interfere with the setting of the resin cement. This difficulty with post cementation using resin cements may also be due to stresses from polymerization shrinkage and problems with adequate access to the root canal. These complicate the formation of high-strength bonds when cementing posts with resin cements.85
Further, it is important to note that abutment teeth cleansed with a prophy cup and flour of pumice exhibit the least amount of residual provisional cement.86 The application of sodium hypochlorite gel after phosphoric acid etching eliminates the inhibitory effect of provisional cement remnants on adhesion between resin cement and dentin.87
A number of manufacturers call their dual-cured cements “all-purpose” luting agents. The problem here is that not all products meet this description. In fact, it has been shown that the choice of a dual-polymerized cement should be based on its intended use, since not all products polymerize adequately in every clinical situation.88 In contrast, another report concluded that dual-cured cements demonstrated the best combination of mechanical and physical properties.89
Since resin cements are tooth-colored, clinicians must be certain to remove excess cement from the margin, as any remaining cement could increase plaque retention. Subclinical cement retention does occur after crown cementation, and this is influenced by a variety of factors, including tooth morphology.90
Discussion
In addition to selection of appropriate materials for a given clinical situation, it is important to use these materials properly. All new chairside employees should be checked for their knowledge of cements, and all should be educated about any new cement introduced to the practice. If a person is having trouble mixing a product by hand, then it should be determined if that product is available in another delivery system, such as an encapsulated form (that can be triturated). Further, when cementing a restoration, occlusion should be checked immediately after seating and corrected if necessary. Some cements set quickly, making removal of the casting difficult. The staff should remember to check the time of trituration before and after use of these products, as the trituration time may be different than that for amalgam.
Clinicians have seen an increase in the number of products that have been packaged in capsules and automix cartridges/syringes (eg, TempAd-vantage, GC America) due to their ease of use. This format requires no spatulation, allows easier cleanup, and ensures equal dispensing of all components. In addition, there is a recent emergence of resin cements that are self etching (eg, Embrace Wetbond Universal Resin Cement, Pulp-dent; RelyX Unicem, 3M ESPE). These products have initially been shown to reduce the amount of postoperative sensitivity associated with cementation.91 Another advantage of these products is ease of use. There are less clinical steps involved for the clinician, as all of the necessary materials are included in the cement itself.
Conclusion
Liner, base, and cement materials are constantly changing. Clinicians have an obligation to keep informed by reading the literature so that the appropriate materials are selected for specific clinical situations.F
References
1. Asa R. Dental materials aren’t what they used to be. AGD Impact. 2004;32:10-13.
2. Weiner RS, Weiner LK, Kugel G. Teaching the uses of bases and liners: a survey of North American dental schools. J Am Dent Assoc. 1996;127:1640-1645.
3. Cox CF, Suzuki S. Re-evaluating pulp protection: calcium hydroxide liners vs. cohesive hybridization. J Am Dent Assoc. 1994;125:823-831.
4. Stanley HR, Going RE, Chauncey HH. Human pulp response to acid pretreatment of dentin and to composite restoration. J Am Dent Assoc. 1975;91:817-825.
5. Cox CF, Keall CL, Keall HJ, et al. Biocompatibility of surface-sealed dental materials against exposed pulps. J Prosthet Dent. 1987;57:1-8.
6. Watts A, Paterson RC. Bacterial contamination as a factor influencing the toxicity of materials to the exposed dental pulp. Oral Surg Oral Med Oral Pathol. 1987;64:466-474.
7. Brannstrom M. Etiology of dentin hypersensitivity. Proc Finn Dent Soc. 1992;88(suppl 1):7-13.
8. Meryon SD. The model cavity method incorporating dentine. Int Endod J. 1988;21:79-84.
9. Camps J, Dejou J, Remusat M, et al. Factors influencing pulpal response to cavity restorations. Dent Mater. 2000;16:432-440.
10. Murray PE, Hafez AA, Smith AJ. Bacterial microleakage and pulp inflammation associated with various restorative materials. Dent Mater. 2002;18:470-478.
11. Going RE. Cavity liners and dentin treatment. J Am Dent Assoc. 1964;69:416-422.
12. Anusavice KJ, Phillips RW, eds. Phillip’s Science of Dental Materials. 11th ed. St Louis, Mo: WB Saunders; 2003.
13. Watts A, Paterson RC. Bacterial contamination as a factor influencing the toxicity of materials to the exposed dental pulp. Oral Surg Oral Med Oral Pathol. 1987;64:466-474.
14. Setien VJ, Cobb DS, Denehy GE, et al. Cavity preparation devices: effect on microleakage of Class V resin-based composite restorations. Am J Dent. 2001;14:157-162.
15. Kugel G. Classification and application of cementation alternatives. Signature. 1997;4:8-11.
16. Farah J. Temporary cements. The Dental Advisor. 1998;15(9):2.
17. Baum L, Phillips RW, Lund MR. Textbook of Operative Dentistry. 3rd ed. Philadelphia, Pa: WB Saunders; 1995:117.
18. Ferracane JL. Materials in Dentistry: Principles and Applications. 2nd ed. New York, NY: Lippincott Williams & Wilkins; 2001:60.
19. Craig RG, Powers JM, eds. Restorative Dental Materials. 11th ed. St Louis, Mo: Mosby; 2001:623.
20. Phillips RW. Skinner’s Science of Dental Materials. 9th ed. Philadelphia, Pa: WB Saunders; 1991:445.
21. Andrews JT, Hembree JH Jr. Marginal leakage of amalgam alloys with high content of copper: a laboratory study. Oper Dent. 1980;5:7-10.
22. Beltran-Aguilar ED, Goldstein JW, Lockwood SA. Fluoride varnishes. A review of their clinical use, cariostatic mechanism, efficacy and safety. J Am Dent Assoc. 2000;131:589-596.
23. von der Fehr FR. Caries prevalence in the Nordic countries. Int Dent J. 1994;44(4 suppl 1):371-378.
24. Lewinstein I, Fuhrer N, Ganor Y. Effect of a fluoride varnish on the margin leakage and retention of luted provisional crowns. J Prosthet Dent. 2003;89:70-75.
25. Shen C, Autio-Gold J. Assessing fluoride concentration uniformity and fluoride release from three varnishes. J Am Dent Assoc. 2002;133:176-182.
26. Yang YK, Chan KC. Effect of varnishes on surface microhardness of basing materials. J Esthet Dent. 1991;3:103-105.
27. Prati C, Fava F, Di Gioia D, et al. Antibacterial effectiveness of dentin bonding systems. Dent Mater. 1993;9:338-343.
28. Smith AJ, Garde C, Cassidy N, et al. Solubilization of dentine extracellular matrix by calcium hydroxide [abstract]. J Dent Res. 1995;74:829.
29. Schuurs AH, Gruythuysen RJ, Wesselink PR. Pulp capping with adhesive resin-based composite vs. calcium hydroxide: a review. Endod Dent Traumatol. 2000;16:240-250.
30. Anderson RW, Powell BJ, Pashley DH. Microleakage of IRM used to restore endodontic access preparations. Endod Dent Traumatol. 1990;6:137-141.
31. Berry CW, Miller BH, Miller AW, et al. Antibacterial activity of dental cements. J Dent Res. 1999;78: Abstract 966.
32. Abou Hashieh I, Camps J, Dejou J, et al. Eugenol diffusion through dentin related to dentin hydraulic conductance. Dent Mater. 1998;14:229-236.
33. Hume WR. The pharmacologic and toxicological properties of zinc oxide-eugenol. J Am Dent Assoc. 1986;113:789-791.
34. Cox CF. Biocompatibility of dental materials in the absence of bacterial infection. Oper Dent. 1987;12:146-152.
35. Weiner RS. Liners, bases, and cements: a solid foundation. Gen Dent. 2002;50:442-446.
36. Arweiler NB, Auschill TM, Reich E. Does pretreatment of cavities effectively promote good marginal adaptation of glass-ionomer cements? J Adhes Dent. 2000;2:289-295.
37. Diaz-Arnold AM, Vargas MA, Haselton DR. Current status of luting agents for fixed prosthodontics. J Prosthet Dent. 1999;81:135-141.
38. Donly KJ, Segura A, Kanellis M, et al. Clinical performance and caries inhibition of resin-modified glass ionomer cement and amalgam restorations. J Am Dent Assoc. 1999;130:1459-1466.
39. Jang KT, Garcia-Godoy F, Donly KJ, et al. Remineralizing effects of glass ionomer restorations on adjacent interproximal caries. ASDC J Dent Child. 2001;68:125-128, 142.
40. Prati C, Fava F, Di Gioia D, et al. Antibacterial effectiveness of dentin bonding systems. Dent Mater. 1993;9:338-343.
41. Tolidis K, Nobecourt A, Randall RC. Effect of resin-modified glass ionomer liner on volumetric polymerization shrinkage of various composites. Dent Mater. 1998;14:417-423.
42. Ricketts D. Management of the deep carious lesion and the vital pulp dentine complex. Br Dent J. 2001;191:606-610.
43. do Nascimento AB, Fontana UF, Teixeira HM, et al. Biocompatibility of a resin-modified glass-ionomer cement applied as pulp capping in human teeth. Am J Dent. 2000;13:28-34.
44. Glasspoole EA, Erickson RL, Davidson CL. Effect of surface treatments on the bond strength of glass ionomers to enamel. Dent Mater. 2002;18:454-462.
45. Awliya WY, Akpata ES. Effect of fluorosis on shear bond strength of glass ionomer-based restorative materials to dentin. J Prosthet Dent. 1999;81:290-294.
46. Periera PN, Okuda M, Sano H, et al. Effect of intrinsic wetness and regional difference on dentin bond strength. Dent Mater. 1999;15:46-53.
47. Konishi N, Watanabe LG, Hilton JF, et al. Dentin shear strength: effect of distance from the pulp. Dent Mater. 2002;18:516-520.
48. Tay FR, Pashley DH. Have dentin adhesives become too hydrophilic? J Can Dent Assoc. 2003;69:726-731.
49. Peutzfeldt A, Vigild M. A survey of the use of dentin-bonding systems in Denmark. Dent Mater. 2001;17:211-216.
50. Imazato S. Antibacterial properties of resin composites and dentin bonding systems. Dent Mater. 2003;19:449-457.
51. Hagan K, Davis A, Belcher M, et al. Microleakage of Class II restorations. J Dent Res. 2003;82: Abstract 933.
52. Vettraino J, Neme A, Pink F, et al. Effect of cavity treatment on in vitro amalgam leakage. J Dent Res. 2003;82: Abstract 1322.
53. Zidan O, Abdel-Keriem U. The effect of amalgam bonding on the stiffness of teeth weakened by cavity preparation. Dent Mater. 2003;19:680-685.
54. Pereira JC, Segala AD, Costa CA. Human pulpal response to direct pulp capping with an adhesive system. Am J Dent. 2000;13:139-147.
55. Chen RS, Liu CC, Tseng WY, et al. Cytotoxicity of three dentin bonding agents on human dental pulp cells [published correction appears in J Dent. 2003;31(7):519]. J Dent. 2003;31(3):223-229.
56. Bayne SC, Thompson JY, Swift EJ Jr, et al. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567-577.
57. Bergenholtz G, Cox CF, Loesche WJ, et al. Bacterial leakage around dental restorations: its effect on the dental pulp. J Oral Pathol. 1982;11:439-450.
58. Gordan V, Shen C, Mjor I. Marginal gap repair with flowable-composite: Where is the weak link? J Dent Res. 2003;82: Abstract 441.
59. Christensen GJ. Direct restorative materials. What goes where? J Am Dent Assoc. 2003;134:1395-1397.
60. CRA Newsletter Clinician’s Guide to Dental Products and Techniques. 2002;26(10):4.
61. CRA Newsletter Clinician’s Guide to Dental Products and Techniques. 2003;27(6):2.
62. Dilts WE, Miller RC, Miranda FJ, et al. The effect of zinc oxide-eugenol on shear bond strengths of selected core/cement combinations. J Prosthet Dent. 1986;55:206-208.
63. Millstein PL, Nathanson D. Effect of eugenol and eugenol cements on cured composite resin. J Prosthet Dent. 1983;50:211-215.
64. Leirskar J, Nordbo H. The effect of zinc oxide-eugenol on the shear bond strength of a commonly used bonding system. Endod Dent Traumatol. 2000;16:265-268.
65. Christensen GJ. Making provisional restorations easy, predictable and economical. J Am Dent Assoc. 2004;135:625-627.
66. Johnson GH, Hazelton LR, Bales DJ, et al. The effect of a resin-based sealer on crown retention for three types of cement. J Prosthet Dent. 2004;91:428-435.
67. Lindquist TJ, Connolly J. In vitro microleakage of luting cements and crown foundation material. J Prosthet Dent. 2001;85:292-298.
68. Coleman AJ, Moses MS, Rickerby HH. Macromolecular leakage beneath full cast crowns: a two-year in vitro investigation. J Prosthet Dent. 2001;85:20-25.
69. White SN. Adhesive cements and cementation. J Calif Dent Assoc. 1993;21:30-37.
70. Li ZC, White SN. Mechanical properties of dental luting cements. J Prosthet Dent. 1999;81:597-609.
71. CRA Newsletter Clinician’s Guide to Dental Products and Techniques. 2004;29:1.
72. Ugarte J, Lagravere M, Revoredo J, et al. Fluoride agent’s uptake effect over two glass ionomer cements and a resin modified glass ionomer cement. J Dent Res. 2003;82: Abstract 938.
73. Gao W, Smales RJ. Fluoride release/uptake of conventional and resin-modified glass ionomers and compomers. J Dent. 2001;29:301-306.
74. Mojon P, Kaltio R, Feduik D, et al. Short-term contamination of luting cements by water and saliva. Dent Mater. 1996;12:83-87.
75. Chuang SF, Jin YT, Tsai PF, et al. Effect of various surface protections on the margin microleakage of resin-modified glass ionomer cements. J Prosthet Dent. 2001;86:309-314.
76. Irie M, Suzuki K. Water storage effect on the marginal seal of resin-modified glass-ionomer restorations. Oper Dent. 1999;24:272-278.
77.CRA Newsletter Clinician’s Guide to Dental Products and Techniques. February 1999.
78. Hersek NE, Canay S. In vivo solubility of three types of luting cement. Quintessence Int. 1996;27:211-216.
79. Nomoto R, McCabe JF. Effect of mixing methods on the compressive strength of glass ionomer cements. J Dent. 2001;29:205-210.
80. Weiner R. Liners, bases, and cements in clinical dentistry: a review and update. Dent Today. Aug 2003;22:88-93.
81. Piemjai M, Miyasaka K, Iwasaki Y, et al. Comparison of microleakage of three acid-base luting cements versus one resin-bonded cement for Class V direct composite inlays. J Prosthet Dent. 2002;88:598-603.
82. Leirskar J, Nordbo H. The effect of zinc oxide-eugenol on the shear bond strength of a commonly used bonding system. Endod Dent Traumatol. 2000;16:265-268.
83. Peutzfeldt A, Asmussen E. Influence of eugenol-containing temporary cement on efficacy of dentin-bonding systems. Eur J Oral Sci. 1999;107:65-69.
84. Hagge M, Wong R, Lindermuth J, et al. Five cements’ post retention following canal obturation using zinc oxide-eugenol sealer. J Dent Res. 2003;82: Abstract 1277.
85. Bouillaguet S, Troesch S, Wataha JC, et al. Microtensile bond strength between adhesive cements and root canal dentin. Dent Mater. 2003;19:199-205.
86. Grasso CA, Caluori DM, Goldstein GR, et al. In vivo evaluation of three cleansing techniques for prepared abutment teeth. J Prosthet Dent. 2002;88:437-441.
87. Watanabe EK, Yatani H, Ishikawa K, et al. Pilot study of conditioner/primer effects on resin-dentin bonding after provisional cement contamination using SEM, energy dispersive x-ray spectroscopy, and bond strength evaluation measures. J Prosthet Dent. 2000;83:349-355.
88. Caughman WF, Chan DC, Rueggeberg FA. Curing potential of dual-polymerizable resin cements in simulated clinical situations. J Prosthet Dent. Jul 2001;86:101-106.
89. Attar N, Tam LE, McComb D. Mechanical and physical properties of contemporary dental luting agents. J Prosthet Dent. 2003;89:127-134.
90. Mitchell CA, Pintado MR, Geary L, et al. Retention of adhesive cement on the tooth surface after crown cementation. J Prosthet Dent. 1999;81:668-677.
91. CRA Newsletter Clinician’s Guide to Dental Products and Techniques. 2004;28(8).
Dr. Weiner received his DMD from Tufts University in 1986. He is a fellow of the Academy of General Dentistry, the American College of Dentists, and the Pierre Fauchard Academy. He maintains a private practice in family and cosmetic dentistry in Millis, Mass, and can be reached at randy@weinerdmd.com.